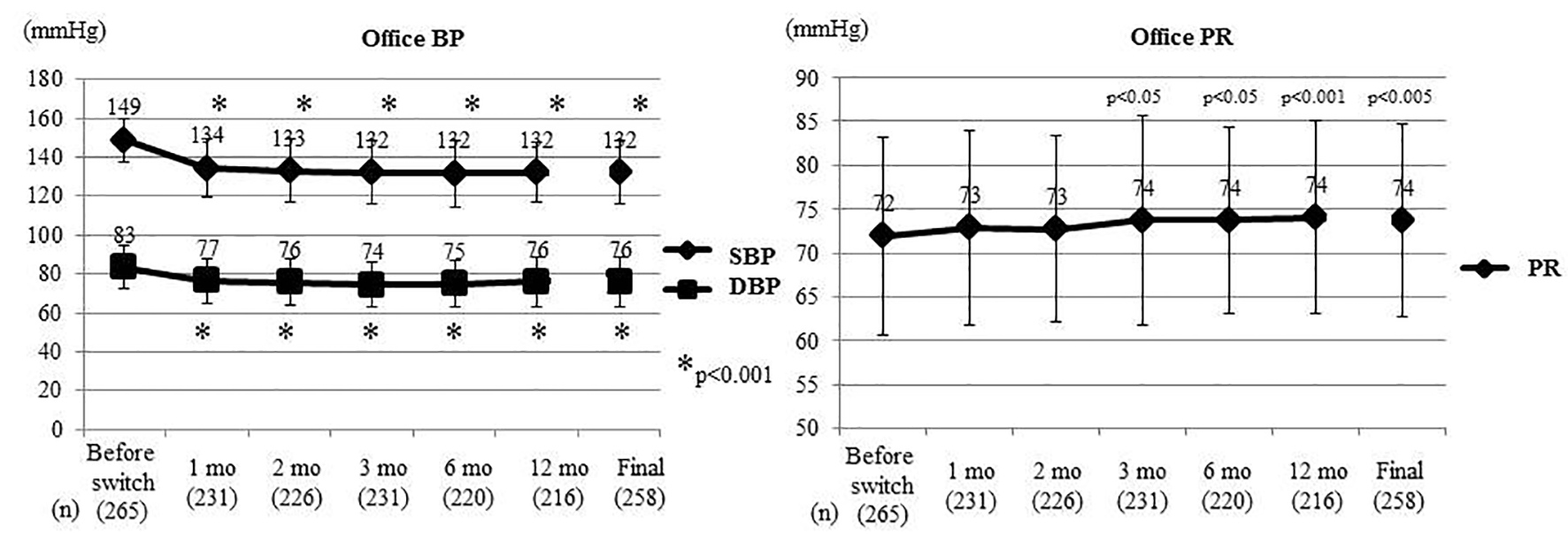
Figure 1. Changes of office BP and PR. At the Final, the last values are shown including patients who experienced deviation. Office BP and PR were compared between before switching and each time point after switching by the t-test. Office SBP and DBP decreased significantly from 1 month after switching, while office PR increased significantly from 3 months. BP: blood pressure; PR: pulse rate; SBP: systolic BP; DBP: diastolic BP; mo: month(s).
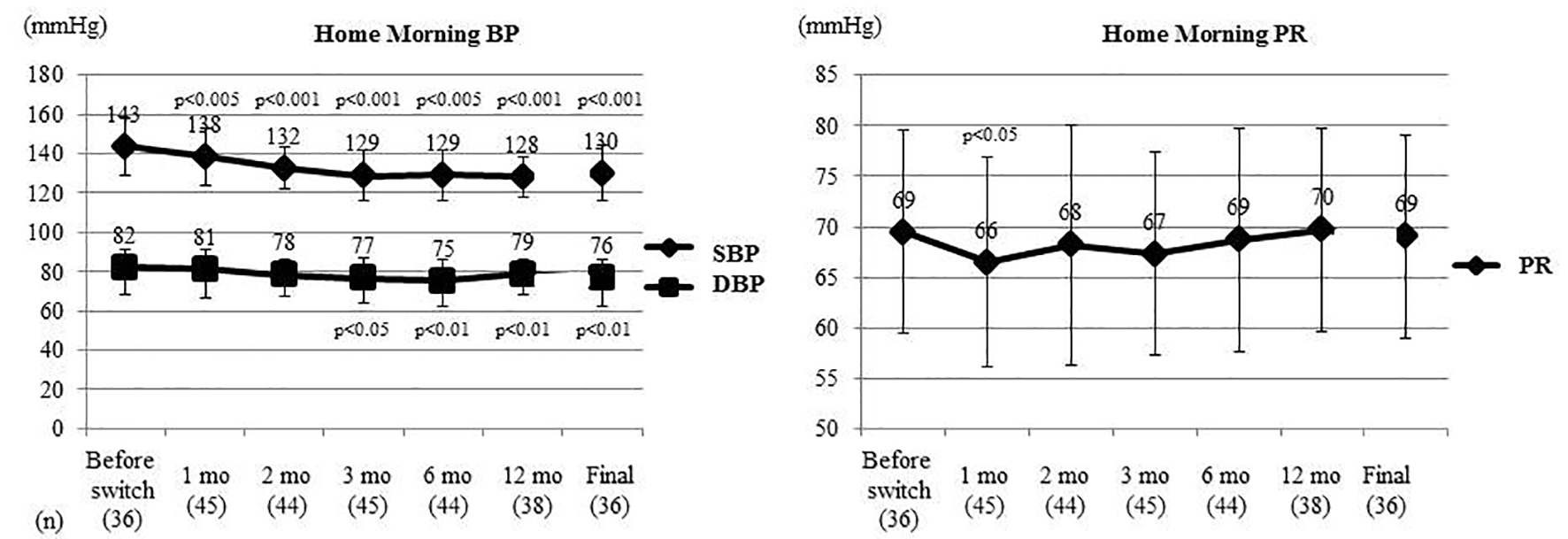
Figure 2. Changes of home morning BP and PR. Home morning BP and PR were compared between before switching and each time point after switching by the t-test. Home morning SBP was significantly decreased from 1 month after switching and home morning DBP was significantly decreased from 3 months. Home morning PR only showed a significant decrease at 1 month. BP: blood pressure; PR: pulse rate; SBP: systolic BP; DBP: diastolic BP; mo: month(s).
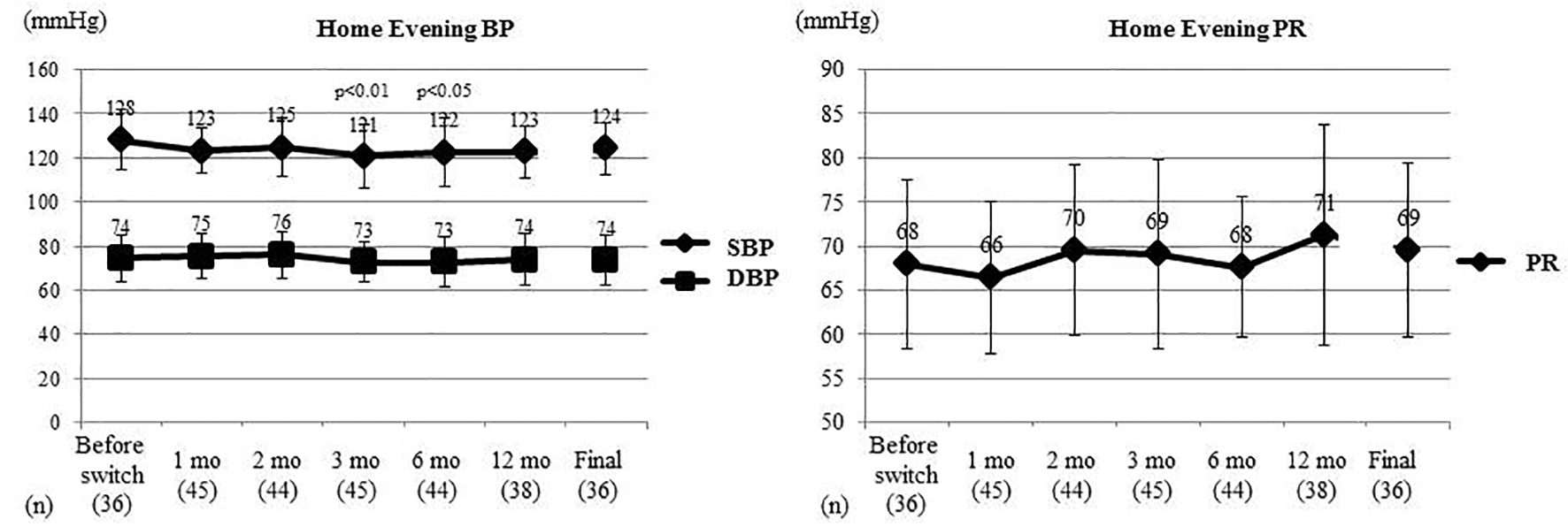
Figure 3. Changes of home evening BP and PR. Home evening BP and PR were compared between before switching and each time point after switching by the t-test. Home evening SBP showed a significant decrease at 3 and 6 months after switching, while home evening DBP and home evening PR did not change significantly. BP: blood pressure; PR: pulse rate; SBP: systolic BP; DBP: diastolic BP; mo: month(s).


