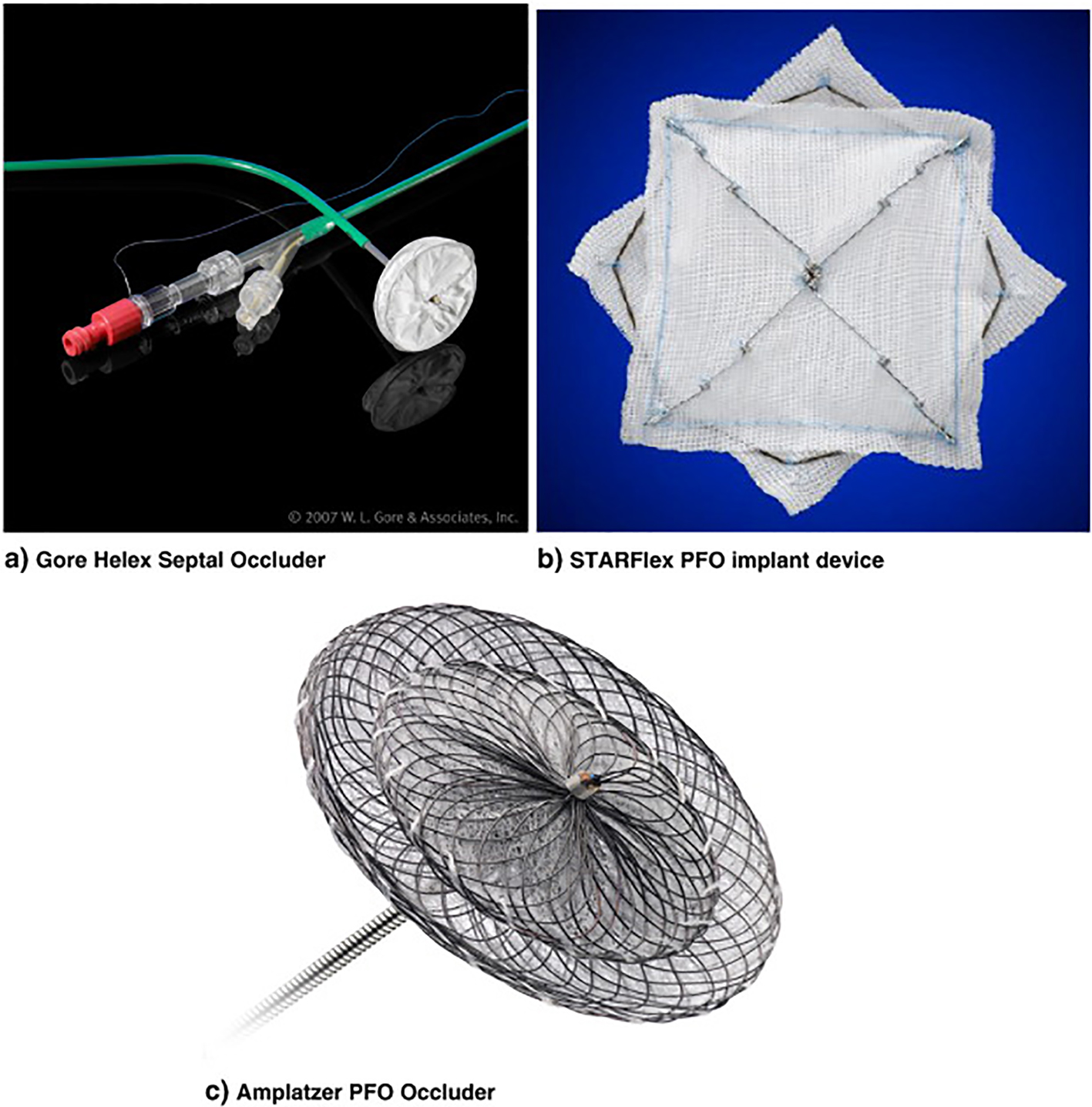
Figure 1. PFO occluding devices. (a) Gore Helex septal occluder; (b) STARFlex PFO implant device; (c) Amplatzer PFO occluder.
| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website http://www.jocmr.org |
Review
Volume 8, Number 5, May 2016, pages 361-366
Unresolved or Contradictory Issues About Management of Patients With Patent Foramen Ovale and Previous Cryptogenic Stroke: Additional Randomized Controlled Trials Are Eagerly Awaited
Figure

Tables
| Study | RCTs only | ITT subset only | Random effect statistical model | Analysis of potential adverse events | Conclusion beneficial |
|---|---|---|---|---|---|
| ITT: intention-to-treat; RCT: randomized controlled trial. | |||||
| Ntaios et al [4] | Yes | No | No | Yes | No overall; yes with Amplatzer |
| Pineda et al [5] | Yes | No | Yes | Yes | No overall; yes as treated |
| Kitsios et al [6] | Yes | No | Yes | No | Inconclusive |
| Riaz et al [7] | Yes | No | Yes | Yes | No overall; yes as treated |
| Hakeem et al [8] | Yes | No | Yes | Yes | Probably |
| Kwong et al [9] | Yes | Yes | Yes | Yes | No |
| Nagaraja et al [10] | Yes | Yes | Yes | Yes | No |
| Rengifo-Moreno et al [11] | Yes | No | No | Yes | Yes |
| Khan et al [12] | Yes | No | No | Yes | Yes |
| Spencer et al [13] | Yes | No | Yes | Yes | No |
| Wolfrum et al [14] | No | No | Yes | Yes | No |
| Capodanno et al [15] | No | Yes | Yes | Yes | No overall; yes with Amplatzer |
| Pandit et al [16] | Yes | Yes | No | No | No overall; yes with Amplatzer |
| Udell et al [17] | Yes | Yes | Yes | Yes | No |
| Li et al [18] | Yes | Yes | Yes | Yes | No |
| Intention to treat | Randomized clinical trials analyzed by the “intention to treat” (ITT) approach provide unbiased comparisons among the treatment groups. “Intention to treat” analyses are done to avoid the effects of crossover and dropout, which may break the random assignment to the treatment groups in a study. ITT analysis provides information about the potential effects of treatment policy rather than on the potential effects of specific treatment. Since it started in the 1960s, the principle of ITT has become widely accepted for the analysis of controlled clinical trials. However, full application of ITT analysis can only be performed where there are complete outcome data for all randomized subjects. |
| As treated | “As treated” analysis has the general idea of comparing the subjects with the treatment regimen that they received. It does not consider which treatment they were assigned for the treatment. |
| Per protocol | The “per protocol” analysis, also known as an “on-treatment” analysis, can only be restricted to the participants who fulfill the protocol in the terms of the eligibility, interventions, and outcome assessment. Indeed, the “per protocol” analysis restricts the comparison of the treatments to the ideal patients, that is, those who adhered perfectly to the clinical trial instructions as stipulated in the protocol. However, by restricting the analysis to a selected patient population, it does not show the practical value of the drug or method that has to be tested. |