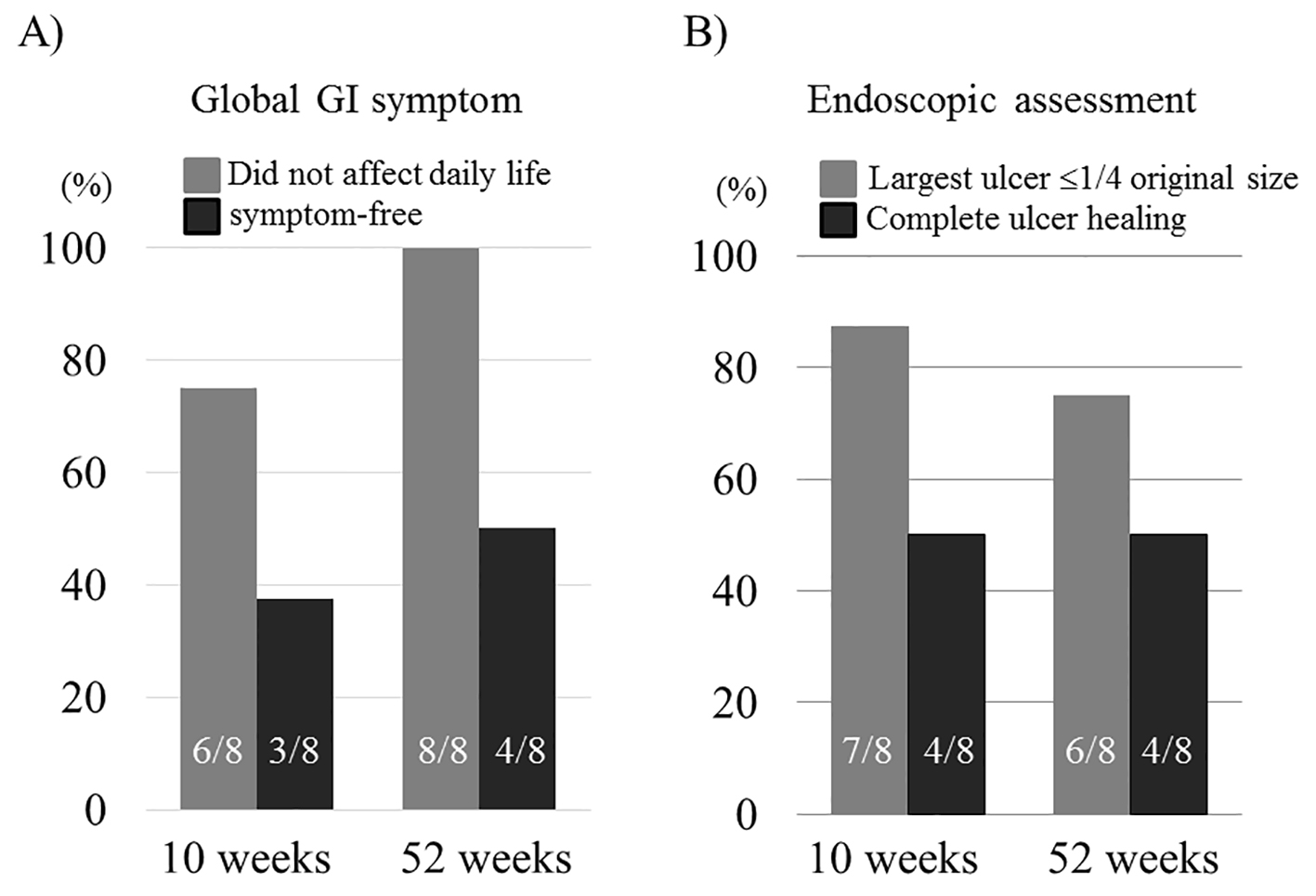
Figure 1. Evolution of the percentage of global GI symptom score 0 or ≤ 1 (A) and endoscopic assessment score 0 or ≤ 1 (B) at 10 and 52 weeks in the eight cases receiving ADA. Data are presented as mean ± SEM.
| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website http://www.jocmr.org |
Case Report
Volume 8, Number 4, April 2016, pages 334-337
Long-Term Efficacy of Adalimumab in Patients With Intestinal Behcet’s Disease: Eight Consecutive Cases
Figure

Tables
| Demography | Number (%) (N = 8) |
|---|---|
| Male sex | 4 (50%) |
| Mean age | 46.6 |
| Smoking (current) | 1 (12.5%) |
| Alcohol | 1 (12.5%) |
| Disease type | |
| Complete/incomplete/suspicious | 0/7/1 |
| GI symptom score | |
| 3 | 5 (62.5%) |
| 4 | 3 (37.5%) |
| Ulcer size at ileocecum | |
| 1 - 2 cm | 2 (25%) |
| 2 - 3 cm | 3 (37.5%) |
| ≥ 3 cm | 3 (37.5%) |
| Concomitant drugs | |
| Mesalazine | 4 (50%) |
| Prednisolone | 4 (50%) |
| Azathioprine | 1 (12.5%) |
| Case No. | Age | Sex | Type | Postoperative recurrence | 0 week | Treated ADA | 10 weeks | Marked improvement | 52 weeks | Marked improvement | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GI symp | Ulcer size | Oral aphtha | CRP | Pretreated | GI symp | End score | CRP | Oral aphtha | GI symp | End score | CRP | Oral aphtha | ||||||||
| Numbers in parentheses indicate doses (mg/day) of concurrent drugs. Asterisk means last observation carried forward because endoscopic score of patient 6 presented with the 32-week score. MLZ: mesalazine; PSL: prednisolone; AZA: azathioprine; CRP: C-reactive protein; ADA: adalimumab; symp: symptom; End score: endoscopic score; failed: failed to achieve clinical response. | ||||||||||||||||||||
| 1 | 65 | M | Incomplete | - | 3 | 3 cm | + | 5.44 | PSL (5) | + | 1 | 1 | 0.37 | - | + | 0 | 2 | 0.43 | - | - |
| 2 | 29 | F | Incomplete | - | 4 | 1 - 2 cm | + | 0.18 | PSL (5) | + | 2 | 0 | 0.10 | - | - | 1 | 0 | 0.14 | - | + |
| 3 | 39 | M | Incomplete | - | 4 | 3 cm | + | 0.38 | PSL (10) | + | 2 | 0 | 0.05 | - | - | 1 | 0 | 0.05 | - | + |
| 4 | 41 | F | Suspicious | - | 4 | 2 - 3 cm | + | 0.20 | PSL (failed), MLZ (2,000) | + | 0 | 1 | 0.05 | - | + | 0 | 1 | 0.03 | - | + |
| 5 | 68 | M | Incomplete | + | 3 | 2 - 3 cm | + | 0.33 | PSL (25), AZA (25) | + | 1 | 1 | 0.08 | - | + | 1 | 1 | 0.15 | - | + |
| 6 | 59 | M | Incomplete | - | 3 | 1 - 2 cm | + | 0.42 | MLZ (3,000) | + | 0 | 0 | 0.04 | + | + | 0 | 0* | 0.03 | + | + |
| 7 | 15 | F | Incomplete | - | 3 | 2 - 3 cm | + | 0.58 | MLZ (2,000) | + | 0 | 0 | 0.04 | + | + | 0 | 0 | 0.07 | + | + |
| 8 | 57 | F | Incomplete | - | 3 | 3 cm | + | 0.34 | MLZ (intolerance) | + | 1 | 2 | 0.65 | + | - | 1 | 2 | 0.37 | + | - |