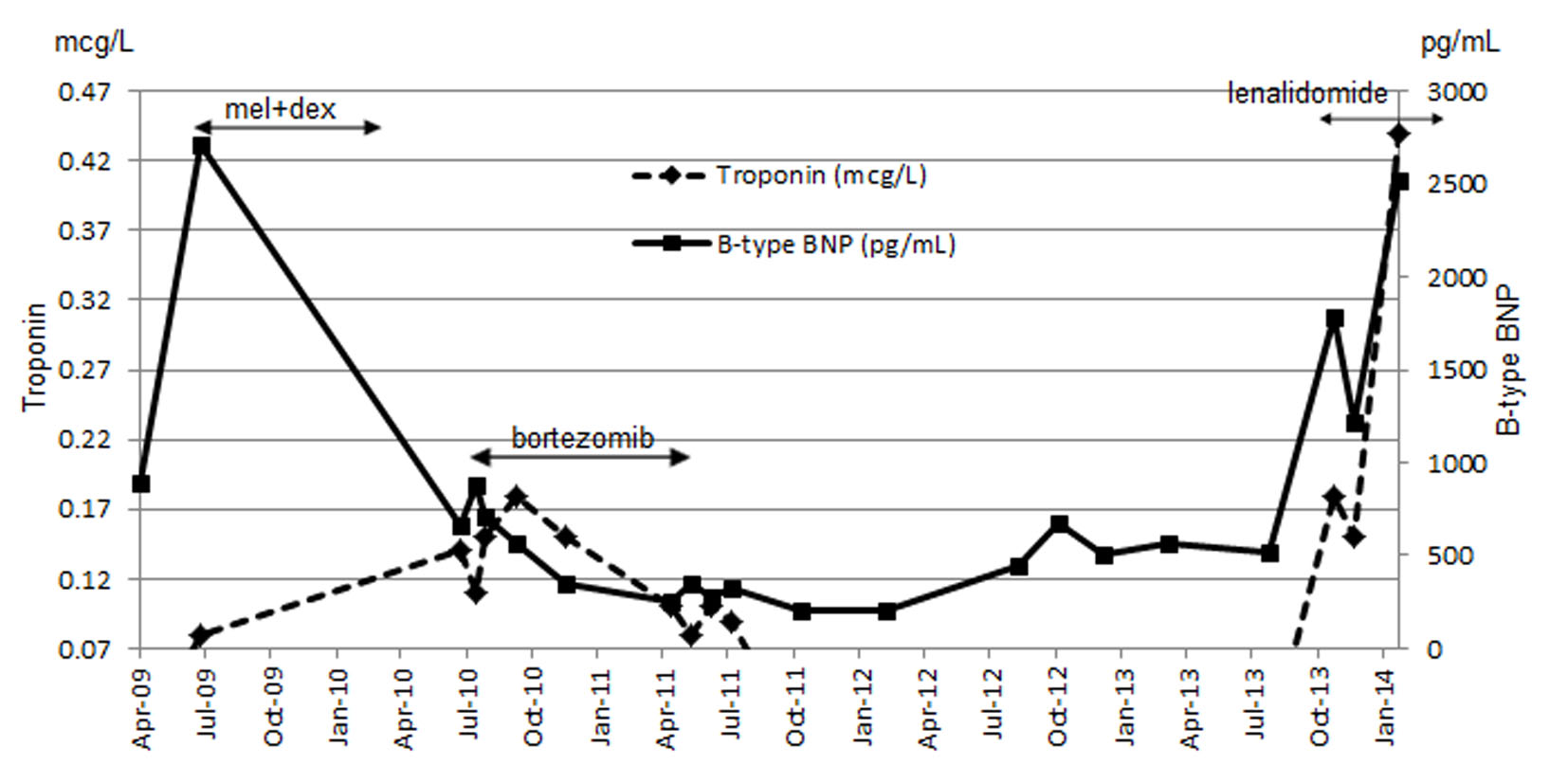
Figure 1. Combined results of troponin and BNP throughout the course of treatment.
| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website http://www.jocmr.org |
Case Report
Volume 7, Number 10, October 2015, pages 807-811
Lenalidomide Desensitization in Systemic Light-Chain Amyloidosis With Multi-Organ Involvement
Figures

Tables
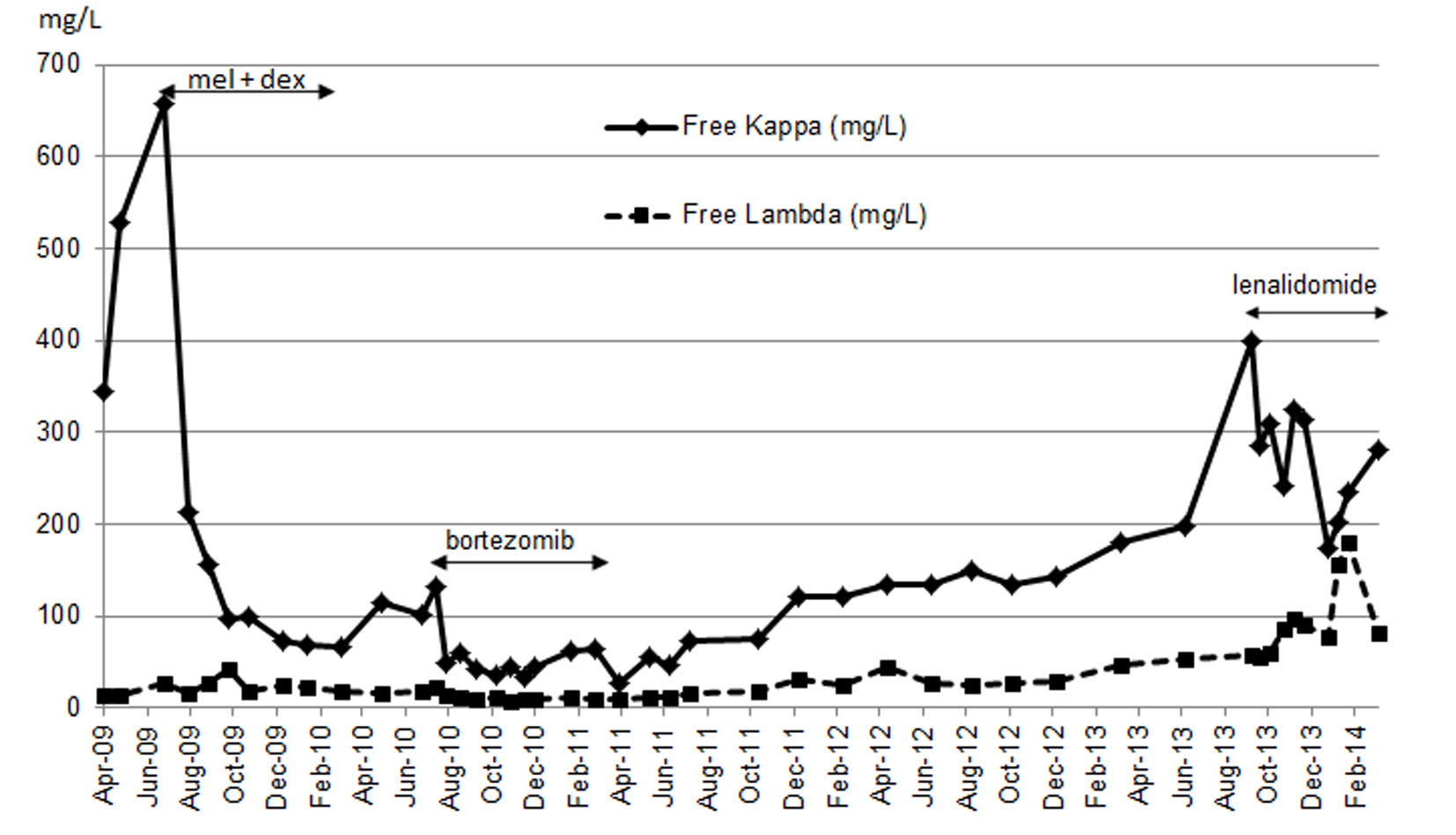
| Chemotherapy | Date | Kappa | Lamda | K/L | dFLC | Hematological response |
|---|---|---|---|---|---|---|
| Mel + dex July 7, 2009 - March 16, 2010 | July 7, 2009 | 657 | 27.3 | 24.07 | 629.7 | PR |
| March 16, 2010 | 65.6 | 17.1 | 3.84 | 48.5 | ||
| Bortezomib July 27, 2010 - May 24, 2011 | July 27, 2010 | 133 | 23.5 | 5.66 | 109.5 | VGPR |
| April 12, 2011 | 27.3 | 9.6 | 2.84 | 17.7 | ||
| Lenalidomide October 15, 2013 - April 19, 2014 | October 15, 2013 | 309.9 | 59.3 | 5.23 | 250.6 | PR |
| January 7, 2014 | 173.1 | 77.6 | 2.23 | 95.5 |
| Dose# | Stock solution concentrations | Dose (mg) | Amount given (mL) by mouth | Time the dose given | BP (mm Hg) | HR per min | RR per min | Temp (°C) | O2 Sat (%) |
|---|---|---|---|---|---|---|---|---|---|
| Baseline | 14:00 | 104/66 | 69 | 16 | 36.6 | 97 | |||
| 1 | Solution D (0.001 mg/mL) | 0.00025 mg | 0.25 | 14:15 | 105/66 | 70 | 16 | 36.9 | 98 |
| 2 | 0.00125 mg | 1.25 | 14:30 | 103/64 | 71 | 16 | 36.5 | 98 | |
| 3 | 0.0025 mg | 2.5 | 14:45 | 104/65 | 71 | 16 | 36.8 | 98 | |
| 4 | Solution C (0.01 mg/mL) | 0.0125 mg | 1.25 | 15:00 | 102/62 | 70 | 16 | 36.9 | 98 |
| 5 | 0.025 mg | 2.5 | 15:15 | 101/62 | 71 | 16 | 37.0 | 99 | |
| 6 | Solution B (0.1 mg/mL) | 0.125 mg | 1.25 | 15:32 | 117/74 | 72 | 16 | 36.7 | 100 |
| 7 | 0.25 mg | 2.5 | 15:45 | 106/69 | 72 | 16 | 37.1 | 100 | |
| 8 | 0.5 mg | 5 | 16:00 | 104/67 | 72 | 16 | 37.0 | 100 | |
| 9 | Solution A (1 mg/mL) | 0.75 mg | 0.75 | 16:15 | 105/66 | 72 | 16 | 37.1 | 100 |
| 10 | 1 mg | 1 | 16:30 | 104/65 | 72 | 16 | 37.1 | 100 | |
| Final vitals | 17:00 | 106/67 | 74 | 16 | 36.5 | 98 | |||
| Observed patients | 17:45 |