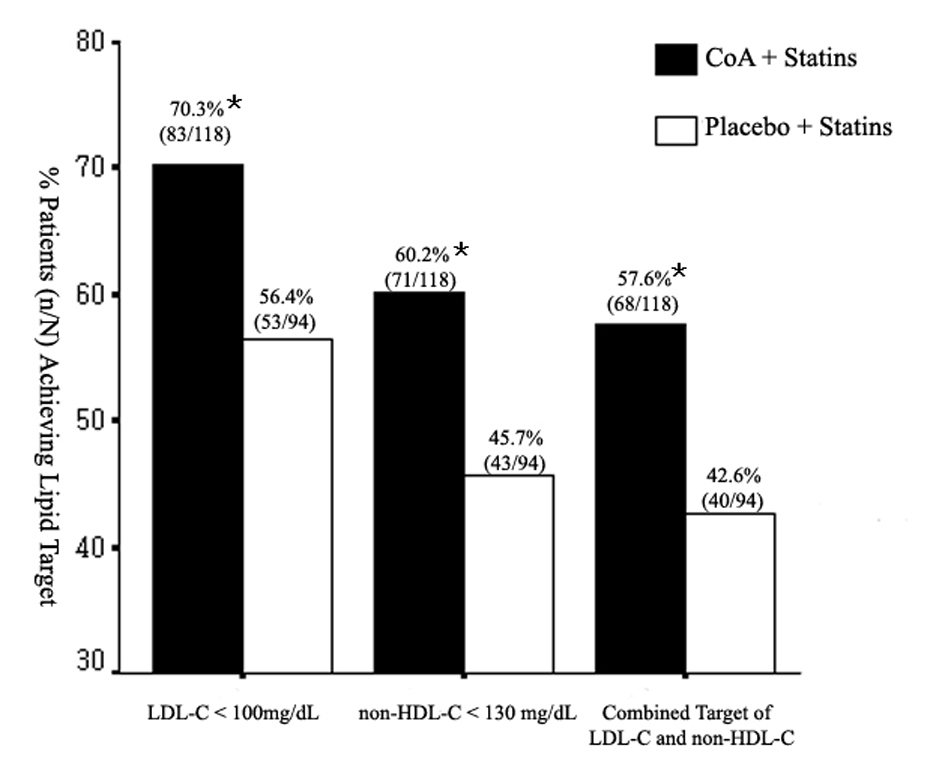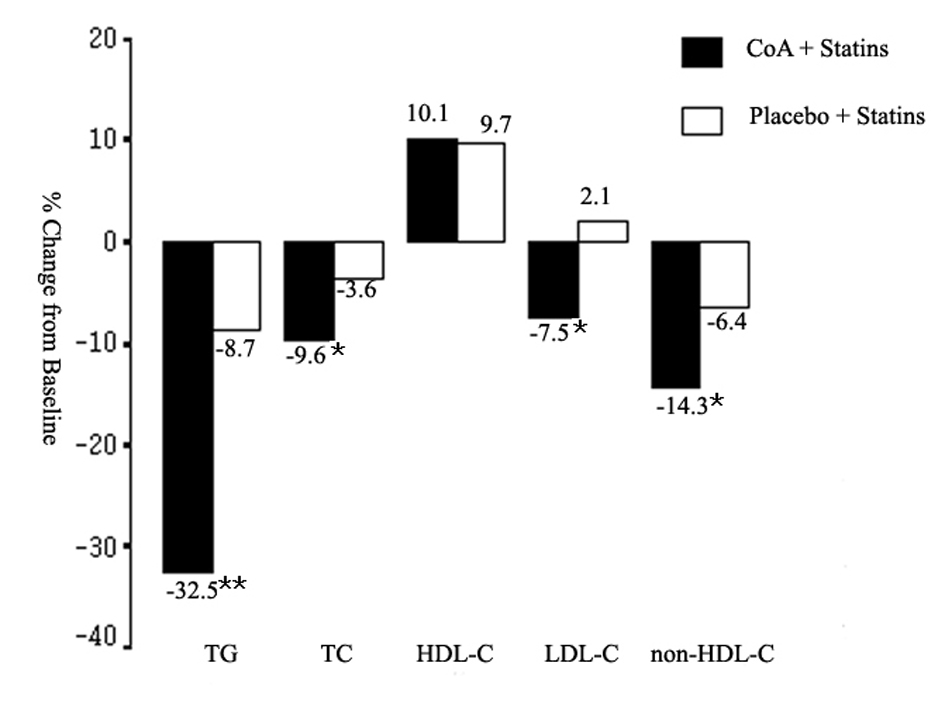
Figure 1. Mean percent change in triglyceride (TG), total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), and non-HDL-C from baseline to the end of study. CoA: coenzyme A. *P < 0.05; **P < 0.01.