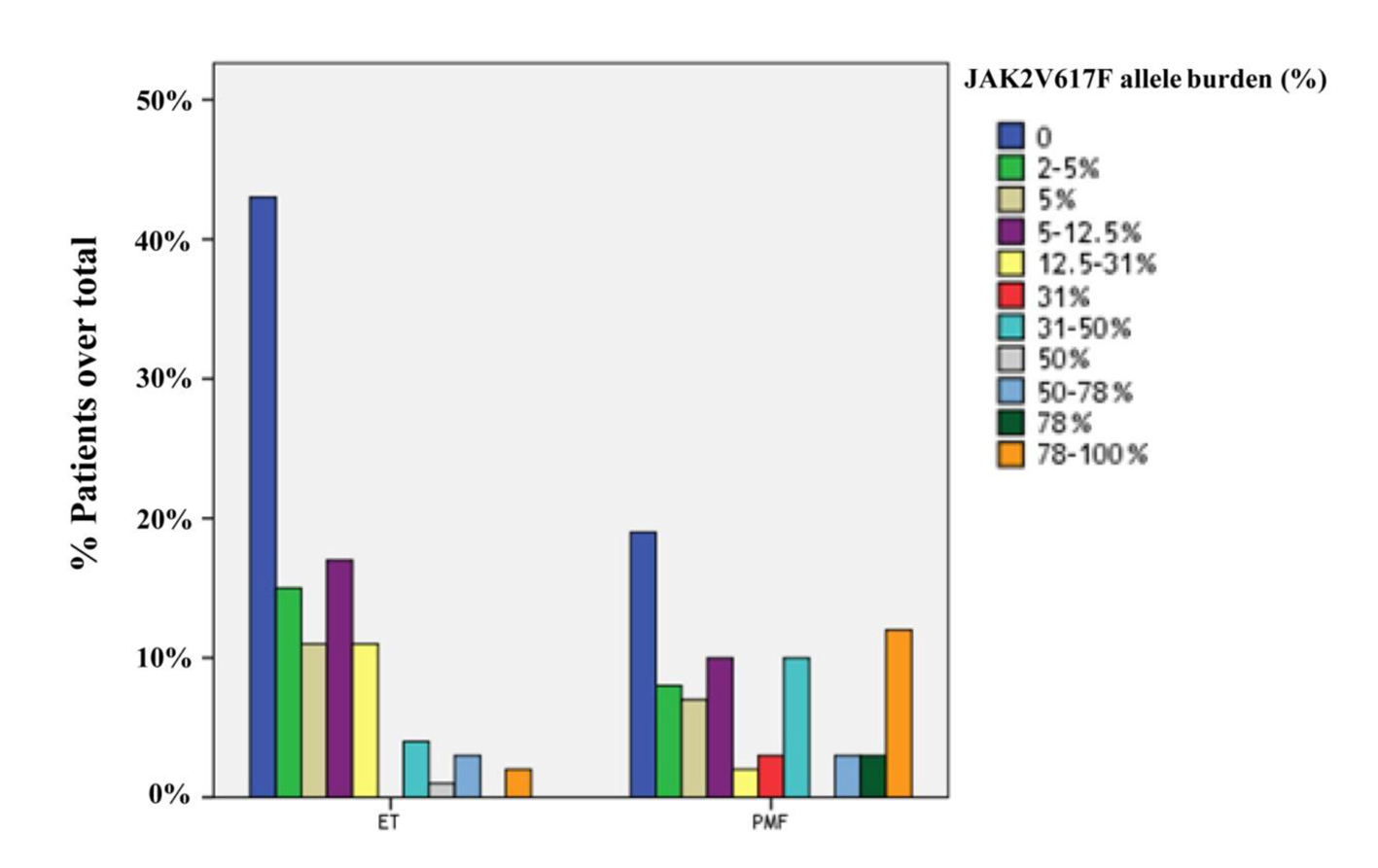
Figure 1. Distribution of ET (n = 107) and PMF (n = 77) patients in quartiles according to the JAK2V617F allele burden. The prevalences of JAK2 wild-type in ET and PMF patients were 40.2% and 24.7%, respectively. The frequency of JAK2V617F-positive patients with mutant allele burden in upper half (allele burden > 50%) was higher in PMF compared to ET (23.4% and 4.7%, respectively; P = 0.001).