| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website http://www.jocmr.org |
Original Article
Volume 10, Number 7, July 2018, pages 562-569
Serum Free Light Chains in Neoplastic Monoclonal Gammopathies: Relative Under-Detection of Lambda Dominant Kappa/Lambda Ratio, and Underproduction of Free Lambda Light Chains, as Compared to Kappa Light Chains, in Patients With Neoplastic Monoclonal Gammopathies
Won Sok Leea, Gurmukh Singha, b
aMedical College of Georgia at Augusta University, Augusta, GA 30912, USA
bCorresponding Author: Gurmukh Singh, Department of Pathology, Medical College of Georgia at Augusta University, 1120 15th Street, BI 2008A, Augusta, GA 30912, USA
Manuscript submitted February 10, 2018, accepted March 2, 2018
Short title: Urine Protein Electrophoresis
doi: https://doi.org/10.14740/jocmr3383w
| Abstract | ▴Top |
Background: Quantitative evaluation of serum free light chains is recommended for the work up of monoclonal gammopathies. Immunoglobulin light chains are generally produced in excess of heavy chains. In patients with monoclonal gammopathy, κ/λ ratio is abnormal less frequently with lambda chain lesions. This study was undertaken to ascertain if the levels of overproduction of the two light chain types and their detection rates are different in patients with neoplastic monoclonal gammopathies.
Methods: Results of serum protein electrophoresis (SPEP), serum protein immunofixation electrophoresis (SIFE), urine protein electrophoresis (UPEP), urine protein immunofixation electrophoresis (UIFE), and serum free light chain assay (SFLCA) in patients with monoclonal gammopathies were examined retrospectively.
Results: The κ/λ ratios were appropriately abnormal more often in kappa chain lesions. Ratios of κ/λ were normal in about 25% of patients with lambda chain lesions in whom free homogenous lambda light chains were detectable in urine. An illustrative case suggests underproduction of free lambda light chains, in some instances.
Conclusions: The lower prevalence of lambda dominant κ/λ ratio in lesions with lambda light chains is estimated to be due to relative under-detection of lambda dominant κ/λ ratio in about 25% of the patients and because lambda chains are not produced in as much excess of heavy chains as are kappa chains, in about 5% of the patients. The results question the medical necessity and clinical usefulness of the serum free light chain assay. UPEP/UIFE is under-utilized.
Keywords: Serum protein electrophoresis; Urine protein electrophoresis; Monoclonal gammopathy
| Introduction | ▴Top |
Plasma cell dyscrasias are a common disorder in adults. Plasma cell myeloma/multiple myeloma is the most common malignancy of the lymphoid system, next to the broad, heterogeneous group of non-Hodgkin lymphomas [1, 2]. Neoplastic plasma cell disorders are classified into three groups, namely, monoclonal gammopathy of undetermined significance (MGUS), smoldering multiple myeloma (SMM) and multiple/plasma cell myeloma (MM) in increasing order of severity, with plasma cell myelomas reflecting a malignant progression of the former [3, 4]. These three entities, namely, MGUS, SMM and MM are being referred to as neoplastic monoclonal gammopathies (NMG). The etiology of MM is not known. Advances in chemotherapy and autologous stem cell transplants (ASCT) have improved the outcomes of treatment; however, plasma cell dyscrasias remain generally incurable [5, 6].
Screening and diagnosis of monoclonal gammopathies (including MGUS, SMM and MM) is generally based on electrophoretic examination of serum and urine proteins, namely serum protein electrophoresis (SPEP)/serum protein immunofixation electrophoresis (SIFE), urine protein electrophoresis (UPEP)/urine protein immunofixation electrophoresis (UIFE), and bone marrow examination [7]. Quantitative laboratory assessment of serum free light chains has been recommended and promoted in the algorithm for diagnosis and monitoring of neoplastic monoclonal gammopathies, despite differences of opinion about the usefulness of serum free light chain assay (SFLCA) [8-12]. The assay for serum free light chains is based on the biological observation that immunoglobulin light chains are produced in excess of the corresponding heavy chains. The excess free light chains can be quantified in serum and are also excreted in urine. Historically, monoclonal/homogenous free light chains were detected in urine by a temperature-dependent precipitation reaction, i.e., Bence Jones proteins. Free light chains are detectable in normal urine as the light chains being of small size, 25 kDa, are freely filtered through the glomerulus. Excess free homogenous/monoclonal light chains in patients with monoclonal gammopathy are also excreted in urine and sometimes produce nephropathy due to precipitation of these proteins in renal tubules, among other mechanisms of renal injury in monoclonal gammopathies [13]. The terms kappa and lambda light chain lesions are meant to include both intact monoclonal immunoglobulins and light chain only lesions, e.g., IgG kappa, as well as kappa chain only lesions.
Serum free kappa and lambda light chains are normally present in a ratio of about 0.26 to 1.65 [14]. In patients with lambda chain producing monoclonal gammopathies, the ratio is depressed and in patients with kappa chain lesions the ratio is elevated. This alteration in ratio has been promoted as a diagnostic tool in the algorithm of plasma cell dyscrasia workup. However, there is a high rate of false positive results in tertiary care patients in general, and those with polyclonal increase in gamma globulins, in particular. In virtually all instances of false positive SFLCA results, in patients without monoclonal gammopathy, there is an excess of kappa chains, and kappa dominant abnormal κ/λ ratio. In tertiary care patients, about 36% of the patients have an abnormal κ/λ ratio without evidence of monoclonal gammopathy. In nearly 90% of these instances the abnormal κ/λ ratio is kappa dominant [10].
In patients with kappa chain monoclonal gammopathies, the κ/λ ratio, is more often observed to be abnormal by being appropriately kappa dominant (true positive), as compared to the incidence of lambda dominant κ/λ ratio (true positive) in patients with lambda chain lesions. The κ/λ ratio is much less often abnormal in patients with lambda chain lesions, i.e., there is a high false negative rate for lambda dominant κ/λ ratio in monoclonal gammopathies associated with lambda chains. For example, the false negative rate for SFLCA results in lambda chain lesions in MGUS, SMM and MM are 89%, 60% and 51%, respectively; the corresponding false negative rates for kappa chain lesions are 60%, 0% and 19%, respectively [11]. The overall excess false negative κ/λ ratio rate for lambda chain lesions, compared to kappa chain lesions, is approximately 30%.
In patients treated with ASCT, the engraftment/healing process is often accompanied by the development of oligoclonal pattern, with or without a detectable neoplastic monoclonal protein. The serum free kappa chains from such oligoclonal patterns sometimes overwhelm the serum content of free light chains by producing a kappa dominant κ/λ ratio, in patients with lambda chain myeloma. Such an aberrant κ/λ ratio was noted in about 15% of the lambda chain myelomas following ASCT, whereas an aberrant lambda dominant κ/λ ratio in patients with kappa chain myelomas was not detected in any case [15].
It has been hypothesized that the high false negative rate for the lambda dominant κ/λ ratio, in patients with lambda chain neoplastic monoclonal gammopathies, may be due to under-detection of lambda light chains. It was suggested that due to the greater tendency of lambda chains, than kappa chains, to dimerize, the target epitopes of the Binding Site antibodies in free dimerized lambda chains may be inaccessible resulting in under-detection of free lambda chains, relative to kappa chains [11]. An alternative explanation could be that lambda chains are not produced in as much excess as are kappa chains and thus result in lower rates of lambda dominant κ/λ ratio in patient with bona fide lambda light chain neoplastic monoclonal gammopathies. A third alternative could be that polyclonal kappa light chains are overproduced in lambda chain monoclonal gammopathies, as is the usual case for tertiary care patients, resulting in a false negative lambda dominant κ/λ ratio. This investigation was undertaken to address the first two alternatives by comparing the results of serum and urine protein electrophoreses with the results of SFLCA.
| Materials and Methods | ▴Top |
This study was conducted at a 480-bed tertiary care, medical school-affiliated medical center with a bone marrow transplantation program, in the Southeastern United States. The protocol was approved by the Institutional Review Board. Data from some of the patients reported in this study were used in earlier publications as well [10, 11, 15].
As described previously, serum and urine protein electrophoreses were carried out using a Helena (Beaumont, Texas) SPIFE 3000 instrument and by using gels procured from Helena. UPEP and UIFE were also carried out with Helena SPIFE 3000. Urine samples were concentrated with Minicon clinical sample concentrators from Millipore. The method achieved a concentration of 5 to 50-fold, depending on the protein concentration in the neat sample. Samples with low protein concentration in the neat state achieved higher levels of volume reduction. Serum free light chains were assayed using an Advia 1800 analyzer, prior to June 2016, and with Optilite analyzer since then, with reagent kits procured from the Binding Site (Birmingham, UK). The laboratory performing these and related tests is accredited by the College of American Pathologists in keeping the standards of Clinical Laboratory Improvement Amendments of 1988.
SPEP/SIFE, UPEP/UIFE, and SFLCA results from January 2010 through September 2017 at this institution were retrospectively reviewed. Data from a total of 482 patients comprising 2,448 observations were examined. Patients with lymphomatous lesions, such as lymphoma and chronic lymphocytic leukemia were excluded. In 193 patients with a monoclonal immunoglobulin, with a total of 279 observations, results of SPEP/SIFE, UPEP/UIFE and SFLCA were available. Of these 193 patients, 175 patients with 249 observations had a diagnosis of MGUS, SMM or MM, i.e., NMG, and evaluable results from SPEP/SIFE, UPEP/UIFE and SFLCA were available to address the questions of this investigation. The remainder of the patients, among the initial 193, had diagnoses of monoclonal immunoglobulins associated with amyloidosis, auto-immune or inflammatory disorders.
Observations in patients with neoplastic monoclonal immunoglobulins, i.e., MGUS, SMM and MM, were screened for results of SPEP/SIFE, UPEP/UIFE and SFLCA. The results were included in the study if all of the observations were within about 2 weeks of one another. Patients with biclonal or triclonal, and non-secretory lesions were excluded, except one case with a biclonal pattern that is presented as an illustrative case but is not included in the numerical data for statistical analysis.
Diagnoses of neoplastic monoclonal gammopathies were arrived at by using prevalent criteria for MGUS, SMM and MM from the findings of SPEP/SIFE, UPEP/UIFE, SFLCA and bone marrow examination as well as other laboratory and imaging studies [6-8].
The data were segregated into three main categories, based on the findings of UPEP/UIFE. It needs to be mentioned that before 2016, only urine samples with more than 15 mg/dL protein were subjected to UIFE analysis at this institution. Starting from mid-2016, all urine specimens with sufficient sample volume were concentrated and analyzed by UIFE. The three UIFE data categories were: 1) UIFE 0, when no monoclonal immunoglobulin or free monoclonal light chains were detected. 2) UIFE 1, when monoclonal free light chains were detected with or without intact monoclonal immunoglobulin. 3) UIFE 2, when only intact monoclonal immunoglobulin was detected. It was understood that free homogenous light chains co-migrating with intact monoclonal immunoglobulin would not be recognized; thus, the number of samples with free homogenous light chain could have been under-estimated.
These three groups were further segregated by the type of light chain lesion, i.e., kappa or lambda light chain. We emphasize that the term kappa or lambda chain lesions includes neoplastic monoclonal gammopathies of intact immunoglobulins with the relevant light chain type.
Further categorization was based on the results of SFLCA. The result was labeled as positive if it was associated with appropriately kappa or lambda dominant κ/λ ratio. For instance, IgG lambda monoclonal gammopathy with the SFLCA revealing a κ/λ ratio lower than 0.26, was identified as a sample positive for lambda dominant κ/λ ratio.
The findings in the various groups were compared by the Chi-square test.
| Results | ▴Top |
Results of SPEP/SIFE, UPEP/UIFE and SFLCA were available in 193 patients with monoclonal immunoglobulins in their sera. After excluding patients with amyloidosis, autoimmune disorders and POEMS etc., 175 patients had 249 evaluable observations. Patients with biclonal lesions were excluded; however, one such patient provided observations relevant to the hypotheses being tested and is presented before the rest of the data.
The findings of SIFE and UIFE from this instructive patient with biclonal gammopathy are displayed in Figure 1. The patient had a dominant IgG lambda monoclonal spike in the serum, at a concentration of about 1.4 g/dL. A slightly anodal, monoclonal IgG kappa band was detectable on SIFE but was not quantifiable. UIFE revealed albumin and monoclonal kappa light chain band but no lambda light chain band. SFLCA revealed a normal κ/λ ratio, albeit with a higher concentration of free kappa light chains than lambda light chains, despite the presence of a monoclonal IgG lambda band of 1.4 g/dL concentration. The concentrations of kappa and lambda serum free light chains were 1.67 mg/L and 1.17 mg/L respectively, with a κ/λ ratio of 1.43. These findings, admittedly in an isolated case, emphasize the lower than expected production of excess free lambda light chains. The normal κ/λ ratio could be due to either under-production of excess free lambda light chains or due to poor detection of lambda chains, as a result of dimerization, or other unknown factors such as excessive production of polyclonal serum free kappa light chains. However, a lack of free homogenous lambda chains in urine strongly suggests relative under-production of free homogenous lambda light chains. Given that albumin, with a molecular weight of 67 kDa, is detectable in urine, but lambda light chains are not, points to the lack of free homogenous lambda light chains in the serum. Even if the lambda light chains had dimerized, the dimeric chains with a molecular weight of about 50 kDa should still have been detectable in urine given a protein of higher molecular weight, albumin, was detectable. This single case could be emblematic of the general situation of lower, excess production of lambda light chains as compared to kappa light chains, vis-a-vis the quantity of heavy chain production, in some patients.
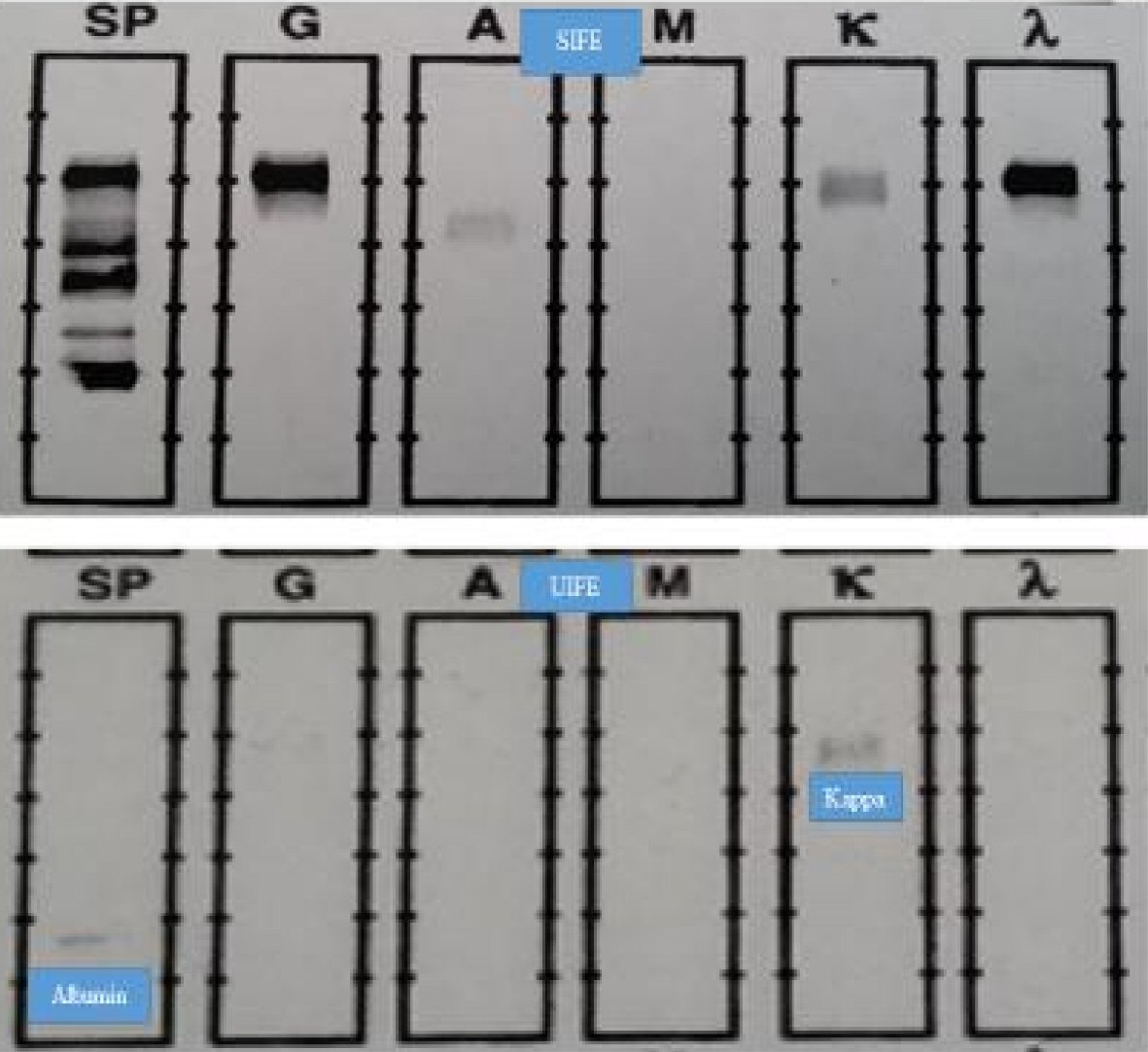 Click for large image | Figure 1. SIFE and UIFE results for the instructive patient with biclonal gammopathy. The results from this patient with biclonal gammopathy are instructive. The lack of lambda dominant κ/λ ratio and absence of monoclonal lambda chains in urine strongly suggest underproduction of excess free lambda light chains. We estimate that out of the total 30% false negative rate for lambda dominant κ/λ ratio, by the SFLCA, about 5% are due to under-production of excess free lambda light chains and about 25% due to under-detection of monoclonal lambda light chains by the Binding Site assay, or due to excess production of polyclonal kappa light chains in tertiary care patients thus resulting in distorted κ/λ ratio in patients with lambda chain lesions. |
Results from the 175 patients with 249 interpretable observations, Table 1, were analyzed with respect to the findings of UIFE. Kappa chain lesions show a kappa dominant κ/λ ratio significantly more often than a lambda dominant κ/λ ratio is observed in samples from patients with lambda chain lesions, in all categories of UIFE findings. The results shown in Table 1, especially the results for UIFE 1 samples, strongly support the relative under-detection of serum free lambda light chains, or effects of excess polyclonal kappa light production in tertiary care patients.
 Click to view | Table 1. Distribution of SFLCA Results of Patients With Monoclonal Gammopathies Categorized by UIFEs |
Tables 2 and 3 show the relative distribution of various primary lesions of neoplastic monoclonal gammopathies by UIFE findings, light chain type of the lesion and SFLCA results. In these and subsequent tables only observations from patients with neoplastic monoclonal gammopathies, i.e., MGUS, SMM and MM were included; specifically, sample with monoclonal immunoglobulins associated with other diagnoses, e.g., amyloid, lymphoma, leukemia, autoimmune disorders etc., were excluded; in contrast to the Table 1, which included the total number of the patients.
 Click to view | Table 2. Relative Distribution of the Neoplastic Monoclonal Gammopathies With Respect to the Categories of UIFE |
 Click to view | Table 3. Distribution of SFLCA Results of Patients With Neoplastic Monoclonal Gammopathies Categorized by UIFEs |
In Table 3, the categories Kappa and Lambda represent the light chain type of the monoclonal immunoglobulin. The titles Neg. and Pos. refer to the appropriate light chain dominant κ/λ ratio, in the serum. Negative represents normal κ/λ ratio or inappropriate light chain dominant κ/λ ratio. Positive represents appropriately dominant κ/λ ratio i.e., a value of < 0.26 for lambda chain lesions and a value > 1.65 for kappa chain lesions. In all three of the UIFE groups, kappa chain lesions demonstrated a kappa dominant κ/λ ratio significantly more often than the incidence of lambda dominant κ/λ ratio in lambda chain lesions.
The observations from patients with kappa or lambda chain primary lesions were segregated by UIFE results as shown in Table 4. The UIFE findings in kappa and lambda chain lesions were not significantly different from each other, with respect to their distribution among the three UIFE groups.
 Click to view | Table 4. Distribution of the Light Chain Type of Lesions of Neoplastic Monoclonal Gammopathies With Respect to their UIFE Categories |
The evaluable 249 observations from neoplastic monoclonal gammopathy patients, for whom SPEP/SIFE, UPEP/UIFE and SFLCA results were available, are compiled in Table 5. In each category of UIFE results, samples from patients with kappa chain lesions, the kappa dominant κ/λ ratio results were significantly more prevalent than lambda-dominant κ/λ ratio results for samples from patients with lambda chain lesions. In Table 5, the results for the UIFE 1 group are particularly illuminating in that in all of the samples from kappa chain lesions with monoclonal light chain in the urine, the κ/λ ratio was kappa dominant. In the corresponding samples from lambda chain lesion, 20% of the samples did not exhibit a lambda dominant κ/λ ratio, indicating that serum free lambda light chains are systematically under-detected by the Binding Site assay, or that the κ/λ ratio is altered by unknown factors to produce a high false negative rate for lambda dominant κ/λ ratio. The findings in the UIFE 0 and UIFE 2 are not amenable to differentiation between under-production and under-detection of serum free lambda light chains.
 Click to view | Table 5. Distribution of SFLCA Results of Patients With Neoplastic Monoclonal Gammopathies (MM + SMM + MGUS) Categorized by UIFEs |
The findings in samples with free homogenous light chains in the urine are particularly remarkable in that all of the samples from kappa chain lesions, in which UIFE displayed free homogenous light chains, with or without accompanying intact immunoglobulin, exhibited a kappa dominant κ/λ ratio. (The P value is a conservative estimate as a value of “1 (one)” had to be entered in place of zero, to calculate the chi square value, in the relevant 2 × 2 table). It is also noteworthy that in about 20% (10 out of 51) of the samples with a detectable free lambda light chains in urine, the serum κ/λ ratio was not lambda dominant in samples from patients with lambda chain lesions. These 10 observations were from 10 different patients. When the observations with lambda dominant κ/λ ratio were edited for unique patients, there were only 31 patients accounting for the 41 observations. Thus, in about 25% of the patients (10 out of 41, Table 6), the lambda light chains were under-detected as indicated by negative lambda dominant κ/λ ratio. The finding strongly argues for systematic relative under-detection of lambda dominant κ/λ ratio. We conclude that out of the total 30% excess false negative rate for lambda dominant κ/λ ratio, (as compared to kappa chain lesions) by the SFLCA, about 5% are due to under-production of excess free lambda light chains and about 25% could be due to under-detection of monoclonal lambda light chains by the Binding Site assay, though unknown factors, such as general over production of polyclonal kappa light chains in tertiary care patients, altering the κ/λ ratio could not be excluded.
 Click to view | Table 6. Distribution of Patients With Neoplastic Monoclonal Gammopathies With SFLCA Results, for UIFE 1 Only |
The finding that a kappa dominant κ/λ ratio had higher incidence in instances in which UIFE did not detect free homogenous light chains is difficult to reconcile but supports the idea that kappa light chains are generally produced in greater excess of heavy chains as compared to lambda light chains, and/or that kappa free light chains are more readily detected by the Binding Site assay for SFLCs. A similar issue of general overproduction of serum free polyclonal kappa light chains in tertiary care patients may explain the high false negative rate for lambda dominant κ/λ ratio in lambda chain monoclonal gammopathy lesions, rather than defective under-detection by the Binding Site assay.
In sum, the findings from samples with detectable free homogenous light chains in the urine favor systematic under-detection of serum free lambda light chains by the Binding Site assay, or an under-detection of lambda dominant κ/λ ratio due to general over production of serum free polyclonal kappa light chains in tertiary care patients, thus affecting the κ/λ ratio. The anecdotal finding from the case report, shown in Figure 1, favors the interpretation of underproduction of excess free lambda light chains being the cause of the low prevalence of lambda dominant κ/λ ratios in some patients with lambda light chain lesions. The results of UIFE 0 and UIFE 2 samples are not readily interpretable but support the notion of under-detection of serum free lambda light chains, relative to kappa light chains. However, the latter set of results do not rule out under-production of excess free lambda light chains, as compared to kappa light chains. Similarly, the third alternative explaining the high false negative rate for lambda dominant κ/λ ratio in lambda chain lesions being due to general over-production of polyclonal kappa light chains in tertiary care patients remains to be investigated.
| Discussion | ▴Top |
The International Myeloma Working Group (IMWG) recommends an algorithm that includes SPEP/SIFE and UPEP/UIFE for the investigation of suspected monoclonal gammopathies [6-8]. While SPEP/SIFE is commonly used screening test, UPEP/UIFE is not performed in many patients. The underutilization of UPEP/UIFE has been further aggravated by the erroneous suggestion that SFLCA can replace UPEP/UIFE [16, 17]. The medical necessity and clinical usefulness of SFLCA has been questioned due to greater than 30% false positive and a more than 30% false negative rate for κ/λ ratio. The rate of false negative results from SFLCA is worse for patients with lambda chain lesions, as exemplified by the fact that about 90% of the MGUS lesions with a lambda chain immunoglobulin type yield normal κ/λ ratio [10, 11]. The higher false negative rate for lambda chain lesions persists for patients with SMM and MM. As alluded to earlier, this apparent under-detection of serum free lambda chains could be due to: 1) A shortcoming of the Binding Site reagents, exacerbated by the tendency of free lambda chains to form dimers. It is conceivable that lambda light chain dimers may not be recognized by the antibody due to inaccessibility of the target epitopes in dimers. 2) Alternatively, the high false negative rate for lambda chain lesions could be due to inherent lower production of serum free lambda light chains, as compared to kappa light chains, for equivalent concentrations of monoclonal immunoglobulins. It has been observed that higher concentrations of IgG lambda may be needed to result in a lambda dominant κ/λ ratio, as compared to IgG kappa monoclonal gammopathies [11, 15]. Both mechanisms (i.e., under-detection and under-production of serum free lambda light chains) are likely to be applicable. 3) A third potential explanation for the high false lambda dominant κ/λ ratio in lambda chain lesions being due to excessive production of free polyclonal kappa light chains in tertiary care patients affecting the κ/λ ratio has been alluded to earlier.
By integrating the findings from earlier publications, and the data from this study, we arrive at the following: 1) The excess (as compared to kappa chain lesions) false negative rate of κ/λ ratio for lambda chain lesions in MGUS is 29% [11]. 2) The excess (as compared to kappa chain lesions) false negative rate of κ/λ ratio for lambda chain lesions in MM is 32% [11]. 3) The excess (as compared to kappa chain lesions) false negative rate of κ/λ ratio for lambda chain lesions in the total pool of neoplastic monoclonal gammopathies is approximately 30% [11]. 4) In 25% of the patients with lambda chain neoplastic monoclonal gammopathies, SFLCA provides a negative κ/λ ratio, in the presence of detectable free homogenous lambda light chains in the urine (Table 6). 5) Under-production of the lambda light chains was demonstrated in an anecdotal case. The rate of under-production is estimated to be about 5%, as explained below.
From the above we surmise that, there is approximately 30% excess false negative rate for κ/λ ratio in lambda chain lesions than in kappa chain lesions. The 30% greater false negative result of κ/λ ratio in lambda chain lesions could be explained by 1) Twenty-five percent rate of under-detection of free homogenous lambda light chains in serum by the Binding Site assay. 2) False negative lambda dominant κ/λ ratio in lambda chain lesions may be due to general over-production of polyclonal kappa chains in tertiary care patients. 3) About 5% rate of under-production of excess free lambda light chains in patients with neoplastic monoclonal gammopathies with lambda chain lesions.
The question of differential over-production of kappa and lambda free immunoglobulin light chains in normal humans and patients with monoclonal gammopathies could be further investigated through multiple methods. For example, in normal subjects as well as patients with monoclonal gammopathies, the total concentration of Hevylite immunoglobulins of kappa and lambda types could be quantified (i.e., the quantity of IgG kappa, IgM kappa and IgA kappa could be determined) and similarly the quantity of all of the lambda chain immunoglobulins could be measured. The same samples could be assayed for the concentration of serum free kappa and lambda light chains. By normalizing the concentrations of serum free light chains to the appropriate Hevylite concentration, it could be ascertained if one type of light chain is produced in greater excess than the other, relative to the production of the corresponding heavy chain [9]. This approach would have to be preceded by documentation that Hevylite assay provides accurate results.
Another approach would be to isolate serum free light chains in given specimens by chromatographic separation of light chains. Monoclonal serum free light chains could be isolated in this manner and the results for kappa and lambda chain specimens compared by normalizing the concentration of the light chains to the relevant monoclonal immunoglobulin concentration.
We chose to address this issue using a different approach, i.e., by querying the data already on file. We reasoned that the presence of free homogenous light chains in the urine of patients with monoclonal gammopathy should reflect the excess production of light chains, as compared to the heavy chains. This approach to assessing the light chain over production would not be dependent on the Binding Site antibody being able to recognize a given light chain, and would obviate any artifact due to dimerization of lambda light chains. We understand that lack of detection of free light chains in urine would not be proof of a lack of excess free light chains in serum, as the monoclonal free light chain in urine could co-migrate with the intact immunoglobulin excreted in urine thereby leading to under-estimation of the rate of detection of excess free monoclonal light chains in urine, and indirectly in the serum. There would also be the issue of the sensitivity of UIFE assay in detecting free homogenous light chains in urine. We addressed these potential concerns by segregating the UIFE results into three groups as described in the methods and results sections. The validity of this approach is supported by the results in instances where free homogenous light chains were detectable in the urine. In all cases in which homogenous kappa light chains were detected in UIFE, the κ/λ ratio was always, appropriately, kappa dominant. The 20% rate of non-lambda dominant serum κ/λ ratio in samples with free homogenous lambda chains in urine and lambda chain neoplastic monoclonal immunoglobulin lesions attests to the under-detection of lambda dominant κ/λ ratio (Table 5). In other words, about 20% of the samples and 25% of the patients with detectable free monoclonal lambda light chains in urine had a negative, i.e., non-lambda dominant κ/λ ratio in serum despite having a lambda chain associated neoplastic monoclonal gammopathy (Tables 5, 6).
The results of UIFE 2 category of urine samples could be further clarified for the presence of free light chains by conducting SDS-PAGE in non-reducing conditions followed by staining for light chains, i.e., Western blotting. Non-reducing SDS-PAGE would separate light chains from intact immunoglobulins based on molecular size and thus identify samples in which light chains could not be identified by UIFE due to co-migration of monoclonal light chains with monoclonal immunoglobulins. However, this method would not distinguish between polyclonal and monoclonal light chains as both have the same molecular weight. A better approach may be to isolate light chains by gel filtration chromatography followed by UIFE to identify monoclonal light chains without interference from intact monoclonal immunoglobulins.
It has been observed and documented that kappa and lambda chain immunoglobulins are produced in a ratio of about 60:40 and the same ratio is seen in plasma cell myelomas [11]. The ratio is more extreme in mice where 95% of the immunoglobulins are kappa chain immunoglobulins. During DNA rearrangement, to generate suitable light-chain coding DNA, the kappa chain DNA is rearranged first, and the process proceeds for immunoglobulin production. If the kappa chain DNA rearrangement is not successful, only then is the lambda chain DNA rearranged. Thus, stochastically there appears to be a biological preference for kappa chain immunoglobulins. It is plausible that kappa chains are produced in greater excess than lambda chains with respect to heavy chain synthesis [18, 19]. This excess production of kappa chains seems to be further magnified in patients with polyclonal gammopathy and tertiary care patients in general.
The results reported here are at variance with reports by others comparing the results of UPEP and serum free light chain assay. Dejoie et al reported greater sensitivity for SFLCA as compared to urine protein analysis in patients with light chain myelomas. They claim better sensitivity for SFLCA than for UIFE even though both had 100% positivity at the outset [20]. The 64% positive result for urine refers to less than 200 mg of the involved monoclonal light chain in 24 h urine, not that monoclonal light chain was detectable in only 64% of the patients [20]. The authors of this paper looked at 113 newly diagnosed light chain myelomas and found that 100% of the patients had an appropriately abnormal κ/λ ratio but only 64% had monoclonal light chain in urine exceeding 200 mg/day. However, they did not point out that 100% of the urine samples were positive for monoclonal light chains by UIFE. Despite 100% positivity by UIFE the authors of this publication recommended against using urine for treatment follow-up without comparing the results of UIFE with SFLCA. The yard stick for noting urine result as being negative was lack of > 200 mg of monoclonal light chain in 24 h urine, not detection of monoclonal light chain in urine by UIFE. In comparing the results of MRD negativity they claim 100% positive predictive value for normal κ/λ ratio. The positive predictive values for UIFE at the end of one and three cycles were 81% and 78%. Our retort to that would be that MRD is a flawed concept for MM, as nearly all patients relapse. We have also reported false positive SFLCA results in patients with oligoclonal pattern during the post-autologous transplant state [15].
This retrospective, observational study has the usual drawbacks of such studies in that data were not collected in a uniform manner resulting in a low rate of UPEP/UIFE studies. A prospective study with organized collection and analysis of samples under standardized conditions may provide more robust results.
The lack of excess production of kappa light chains, relative to heavy chains, and under-detection of kappa light chains by the Binding Site assay, if any, needs to be investigated.
Conclusions
There is systematic under-detection of lambda dominant κ/λ ratio. In about 25% of the patients, with false negative κ/λ ratio, under-detection of the serum free lambda light chains may account for the false negative SFLCA result, as documented by the presence of monoclonal lambda light chains in urine. The excess production of polyclonal kappa chains in patients with lambda chain monoclonal gammopathy altering the κ/λ ratio remains to be investigated. It is likely that kappa chains are biologically produced in greater excess than lambda chains, as compared to immunoglobulin heavy chains, in neoplastic monoclonal gammopathies. The under-production of excess lambda free light chains in patients with neoplastic monoclonal gammopathy, accounts for an estimated 5% false negative κ/λ ratio out of a total of 30% false negative rate. UPEP is grossly underutilized in investigation of monoclonal gammopathies. The medical necessity and clinical usefulness of serum free light chain assay is questionable in most routine clinical circumstances.
Acknowledgments
Shikhar Vyas, MD, kindly assisted with the illustrations. Review of the manuscript by Ronni Bollag, MD, PhD and Wendy Bollag PhD is gratefully acknowledged. The technical assistance of Ms. Constance Johnson, Ms. Kathy Graham and, Ms. Renee Arthur is acknowledged with gratitude.
| References | ▴Top |
- Howlader N, Noone AM, Krapcho M, Miller D, Bishop K, Altekruse SF, Kosary CL, et al. (eds). SEER Cancer Statistics Review, 1975-2013, National Cancer Institute. Bethesda, MD, http://seer.cancer.gov/csr/1975_2013/, based on November 2015 SEER data submission, posted to the SEER web site, April 2016.
- Kyle RA, Rajkumar SV. Multiple myeloma. Blood. 2008;111(6):2962-2972.
doi pubmed - Kyle RA, Rajkumar SV. Monoclonal gammopathy of undetermined significance. Clin Lymphoma Myeloma. 2005;6(2):102-114.
doi pubmed - Kyle RA, Remstein ED, Therneau TM, Dispenzieri A, Kurtin PJ, Hodnefield JM, Larson DR, et al. Clinical course and prognosis of smoldering (asymptomatic) multiple myeloma. N Engl J Med. 2007;356(25):2582-2590.
doi pubmed - Rajkumar SV, Kumar S. Multiple myeloma: diagnosis and treatment. Mayo Clin Proc. 2016;91(1):101-119.
doi pubmed - Landgren O, Rajkumar SV. New developments in diagnosis, prognosis, and assessment of response in multiple myeloma. Clin Cancer Res. 2016;22(22):5428-5433.
doi pubmed - Kumar S, Paiva B, Anderson KC, Durie B, Landgren O, Moreau P, Munshi N, et al. International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol. 2016;17(8):e328-e346.
doi - Dimopoulos M, Kyle R, Fermand JP, Rajkumar SV, San Miguel J, Chanan-Khan A, Ludwig H, et al. Consensus recommendations for standard investigative workup: report of the International Myeloma Workshop Consensus Panel 3. Blood. 2011;117(18):4701-4705.
doi pubmed - Bradwell A. Serum Free Light Chain Analysis plus Hevylite, 7 ed. Birmingham, UK: The Binding Site Group Ltd. 2015.
- Singh G. Serum free light chain assay and kappa/lambda ratio performance in patients without monoclonal gammopathies: high false-positive rate. Am J Clin Pathol. 2016;146(2):207-214.
doi pubmed - Singh G. Serum free light chain assay and kappa/lambda ratio: performance in patients with monoclonal gammopathy-high false negative rate for kappa/lambda ratio. J Clin Med Res. 2017;9(1):46-57.
doi pubmed - Rao M, Lamont J, Chan J, et al. Effective health care program. Future research needs paper number 23. Serum free light chain analysis for the diagnosis, management, and prognosis of plasma cell dyscrasias: future research needs. AHRQ Publication No. 12-EHC135-EF. 2012.
- Herrera GA. Renal manifestations of plasma cell dyscrasias: an appraisal from the patients' bedside to the research laboratory. Ann Diagn Pathol. 2000;4(3):174-200.
doi - Katzmann JA, Clark RJ, Abraham RS, Bryant S, Lymp JF, Bradwell AR, Kyle RA. Serum reference intervals and diagnostic ranges for free kappa and free lambda immunoglobulin light chains: relative sensitivity for detection of monoclonal light chains. Clin Chem. 2002;48(9):1437-1444.
pubmed - Singh G. Oligoclonal pattern/abnormal protein bands in post-treatment plasma cell myeloma patients: implications for protein electrophoresis and serum free light chain assay results. J Clin Med Res. 2017;9(8):671-679.
doi pubmed - Katzmann JA, Kyle RA, Benson J, Larson DR, Snyder MR, Lust JA, Rajkumar SV, et al. Screening panels for detection of monoclonal gammopathies. Clin Chem. 2009;55(8):1517-1522.
doi pubmed - Katzmann JA, Dispenzieri A, Kyle RA, Snyder MR, Plevak MF, Larson DR, Abraham RS, et al. Elimination of the need for urine studies in the screening algorithm for monoclonal gammopathies by using serum immunofixation and free light chain assays. Mayo Clin Proc. 2006;81(12):1575-1578.
doi pubmed - Janeway CA Jr, Travers P, Walport M. The generation of diversity in immunoglobulins. Immunobiology; the immune system in health and disease. New York Garland Science. 2001.
- Perfetti V, Vignarelli MC, Palladini G, Navazza V, Giachino C, Merlini G. Insights into the regulation of immunoglobulin light chain gene rearrangements via analysis of the kappa light chain locus in lambda myeloma. Immunology. 2004;112(3):420-427.
doi pubmed - Dejoie T, Corre J, Caillon H, Hulin C, Perrot A, Caillot D, Boyle E, et al. Serum free light chains, not urine specimens, should be used to evaluate response in light-chain multiple myeloma. Blood. 2016;128(25):2941-2948.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Medicine Research is published by Elmer Press Inc.











