| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website http://www.jocmr.org |
Review
Volume 9, Number 11, November 2017, pages 891-899
C-Reactive Protein as an Independent Cardiovascular Risk Predictor in HIV+ Patients: A Focused Review of Published Studies
Tarvinder S. Gilotraa, c, Stephen A. Geracia, b
aDepartment of Internal Medicine, Quillen College of Medicine, East Tennessee State University, Johnson City, TN, USA
bDivision of Cardiology, Quillen College of Medicine, East Tennessee State University, Johnson City, TN, USA
cCorresponding Author: Tarvinder S. Gilotra, Department of Internal Medicine, Quillen College of Medicine, PO Box 70622, Johnson City, TN 37614, USA
Manuscript submitted August 14, 2017, accepted September 8, 2017
Short title: CRP and Cardiac Events in HIV+ Patients
doi: https://doi.org/10.14740/jocmr3154w
| Abstract | ▴Top |
Patients infected with the human immunodeficiency virus (HIV+) are living longer and at heightened risk for developing cardiovascular events (CVEs). Commonly used prediction tools appear to misrepresent their CVE risk to varying degrees and in varying directions. Inclusion of markers of cellular infection, chronic immune activation and/or systemic inflammation into risk models might provide better predictive accuracy. Observational studies assessing the relationship of high-sensitivity C-reactive protein (hs-CRP) to CVE in HIV+ patients have reported inconsistent findings. This review of published studies attempted to determine if the available evidence supports its potential use in new models for stable, treated HIV+ patients. We searched the PubMed database using keywords and combinations of “HIV” AND “cardiovascular risk” AND “CRP”. Papers presenting original analyses, associating hs-CRP concentration as an independent variable to hard cardiovascular outcomes (myocardial infarction and cardiovascular death), or to hard CVE as part of a composite endpoint, were included. Five observational studies met inclusion/exclusion criteria for review. Three papers identified an association between elevated hs-CRP and CVE, while two others failed to find any significant association. All reports were heterogeneous in terms of independent variables, controls, and designs. The larger and more rigorous studies, employing higher rates of confounder controls and more objective endpoints in their composites, showed positive associations. Though not conclusive, the preponderance of the evidence at this time supports CRP as a potentially valuable factor to be studied in prospective cardiovascular risk prediction investigations in HIV+ patients.
Keywords: Cardiovascular disease; Assessment risk; Cardiovascular models; HIV-acquired immunodeficiency syndrome; C-reactive protein
| Introduction | ▴Top |
Human immunodeficiency virus-infected (HIV+) patients are at heightened risk for developing cardiovascular events (CVEs) [1-5]. Potential explanatory mechanisms include traditional cardiovascular (CV) risk factors (perhaps with magnified effect and/or increased prevalence in this population) [6-8], more frequent fat redistribution/metabolic syndromes [9-12], antiretroviral therapy (ART) influences on atherosclerosis [13-16], and consequences of chronic inflammation and coagulation system activation associated with HIV infection itself [17-20]. Existing traditional risk prediction tools (Framingham risk score (FRS) [21], SCORE [22], ASCVD pooled cohort [23], PROCAM [24], Rama-EGAT [25], and others) appear to underestimate or overestimate CVE risk in HIV patients, particularly at break points of therapy indications, and in inconsistent directions depending upon risk category [8, 26]. Some explanations for this discordance are that the equations were developed for broad, general populations (with unknown and undocumented numbers of HIV+ individuals), and only for people > 40 years old; none included inflammatory or coagulation biomarkers or HIV-specific variables (such as CD4+ cell count, or use, duration and regimen of ART). For most models, calibration studies for an HIV+ population have not been reported, while for some, the HIV+ populations were unique (country, sex, etc.) and insufficiently generalizable to the overall HIV+ population. Model disagreements appear to originate in selection of the validation population and the endpoints identified for prediction, which vary significantly. Differences also stem from inclusion of race and family history as data input variables in some equations but not in others.
The novel adverse effects of anti-HIV drugs (D:A:D) [27] risk assessment tool incorporating CD4+ cell counts and exposures to select ART appear to predict more accurately the cardiovascular disease (CVD) risk compared to the FRS recalibrated for HIV+ cohorts in the United States [28]. However, the D:A:D model predicts risk over only a 5-year period, compared to ASCVD and other equations that estimate 10-year risk. Also, a key factor in the D:A:D model (abacavir exposure) has now been confirmed not to influence the myocardial infarction (MI) rate by the US Food and Drug Administration. Furthermore, two separate models incorporating HIV-specific variables into the ASCVD pooled cohort equations underestimated MI risk at the lower end of the spectrum and overestimated risk in higher risk patients (particularly in women and black men) [29]. The under-prediction will likely worsen if other endpoints of the ASCVD pooled cohort (such as CVD mortality and stroke) are added to the outcome events. Hence, it appears we remain far from an optimal CVE risk prediction tool for HIV+ patients.
Systemic inflammation and chronic immune activation resulting from HIV infection may play a more important causative role in CVE. Inflammation is suggested by elevated plasma concentrations of high-sensitivity C-reactive protein (hs-CRP) in HIV+ patients, especially in those who are treated with ART, compared to healthy controls [18, 19, 30]. Hs-CRP has been one of the most studied inflammatory biomarkers in association with CVD and CVE in the general population. The JUstification for the use of statin in Prevention: an Interventional Trial Evaluating Rosuvastatin (JUPITER) study suggested that elevated hs-CRP concentrations add predictive accuracy to traditional risk factors among intermediate-risk persons in the general population [31].
The addition of hs-CRP to total and high-density lipoprotein (HDL) cholesterol improved CVD risk prediction in otherwise healthy men [32]. In a prospective trial on females [33], hs-CRP was shown to be an independent predictor of MI and stroke in apparently healthy women. Based on these data, separate Reynolds risk scores [34, 35] were designed for men and women that appear more accurate than conventional risk algorithms, particularly for women. Observational studies assessing the ability of hs-CRP to add predictive power in an HIV+ population, however, have reported inconsistent results.
This paper presents a focused review of published studies to determine if hs-CRP is independently associated with elevated risk of hard CVE events in HIV+ patients, and whether inclusion of this biomarker into a predictive model might improve accuracy in estimating major CVE in stable, treated HIV+ patients.
| Literature Search Methods | ▴Top |
The authors searched the PubMed database using keywords “HIV” AND “cardiovascular risk” AND “CRP” (non-MeSH) to maximize the search yield. We included articles with publication dates from database origination through the search date (February 16, 2017). Only full manuscript, English language papers presenting original analyses of data with participant numbers sufficient to permit formal statistical comparisons were included. Studies evaluating the association of hs-CRP plasma concentration as a discreet independent variable to hard CV outcomes (myocardial infarction (MI) and cardiovascular mortality), or to composite outcomes which included hard events, in univariate and, when provided, multivariate analyses were included. Papers that reported on any association of hs-CRP only to outcomes other than hard CV events (e.g., CVD surrogates, CVD risk, other CVD biomarkers, all-cause mortality, and other CVD risk factors), were not included. Bibliographies of identified articles were reviewed to add additional relevant studies missed by the inquiry. Studies that exclusively examined only subpopulations of HIV+ patients (e.g., Hispanic, black, female, or pediatric patients only) were excluded, as were duplicate papers, studies with sample size less than 90, interim reports of active trials, reviews, case reports, opinions and commentaries, and editorials. Finally, when two papers were identified that performed similar analyses on the same data (either exclusively or as part of a pooled data analysis), the duplicate paper was excluded (Fig. 1).
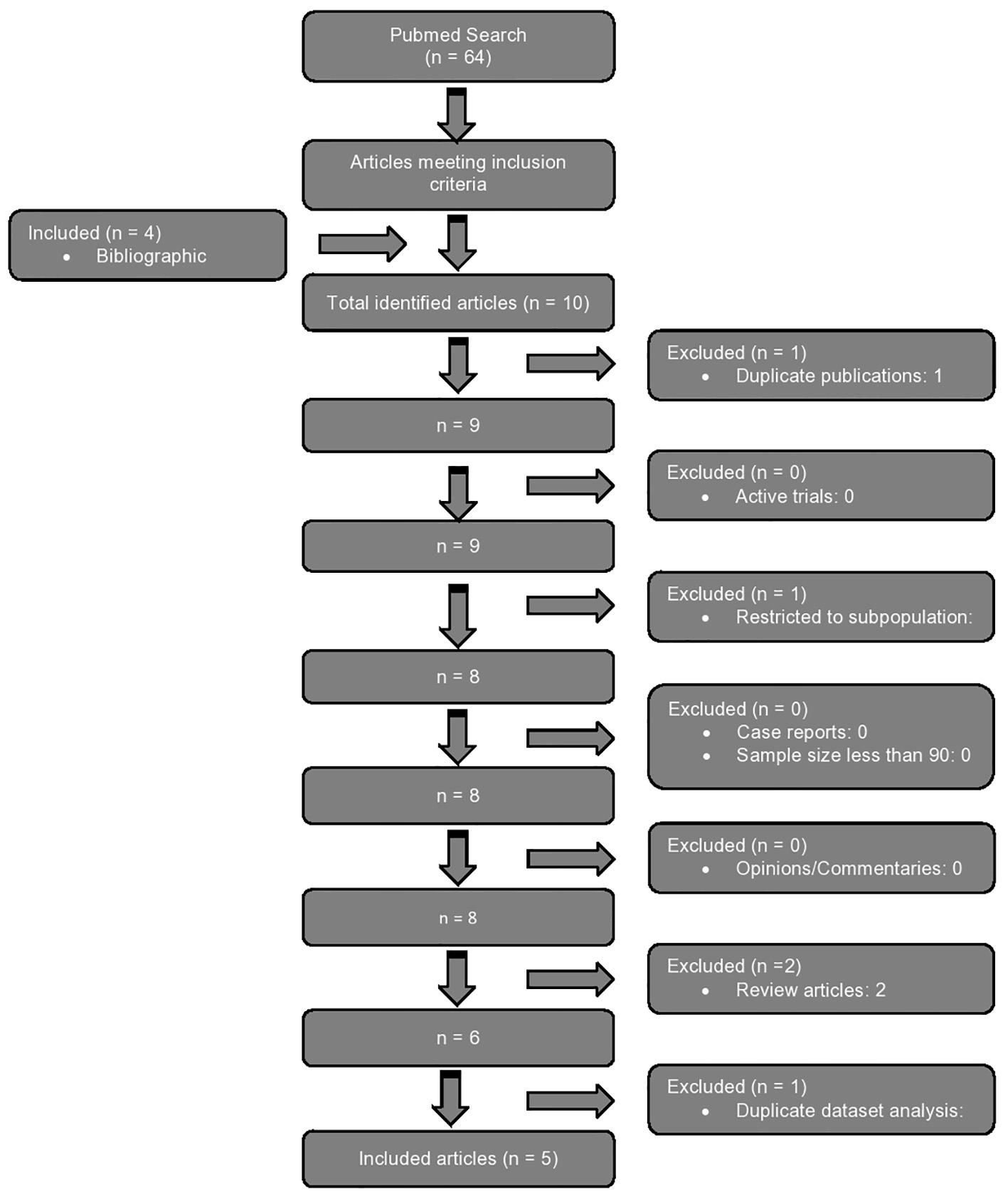 Click for large image | Figure 1. Flow diagram demonstrating step-wise application of inclusion and exclusion criteria to arrive at included articles. |
| Results | ▴Top |
The raw search using the above terms resulted in 64 articles, out of which six papers met the inclusion criteria. Four additional articles were added from bibliographies of identified articles (totally 10 articles). All identified studies examined hs-CRP in addition to other biomarkers for independent associations with hard CV outcomes in multivariate analyses. Duplicate publications (one), studies restricted to a discreet subpopulation of HIV+ patients (one), and review articles (two) were excluded. There were no papers with sample sizes less than 90, case reports, opinions or commentaries, or ongoing trials among the selected articles. Finally, one report with a duplicate data analysis of the same datasets (subset) was excluded. This left five articles that qualified for comparative review (Table 1 [36-40]). Four studies were retrospective and one prospective.
 Click to view | Table 1. Association of CVE, CRP and Other Risk Factors |
Studies concluding a positive association
Triant et al [36] performed a retrospective cohort analysis of 70,357 patients (487 HIV+) with an available hs-CRP plasma level from the Research Patient Data Registry at the Massachusetts General and Brigham and Women’s Hospitals in Boston. Registry data from 1997 through 2006 from patients aged 18 - 84 years were included. The hs-CRP collection times were standardized (with < 3 years between sampling and index event/close of follow-up in primary analyses) among all participants, with patients excluded if their hs-CRP measurement was made only after a coronary event. Classical risk factors were more frequent among HIV+ than among non-infected patients. The presence of HIV itself associated independently with a higher rate of subsequent MIs (compared to uninfected controls). The investigators reported a positive association of elevated CRP concentrations with acute MI in HIV+ registry patients (odds ratio (OR): 2.50, 95% confidence interval (CI): 2.26 - 2.77) independent of traditional CV risk factors, use of statins, or use of nucleoside reverse transcriptase inhibitors (NRTIs) or non-NRTI (NNRTIs). There were no differences in smoking frequency, statin use, or NRTI or NNRTI use between cohorts with elevated vs. normal CRP levels. The multivariate model adjusted for age, sex, race, hypertension, diabetes, and dyslipidemia. There were no reported associations of prior history of CVD, body mass index (BMI), family history of premature coronary disease, duration of HIV infection, acquired immunodeficiency syndrome (AIDS) diagnosis, or ART duration with hs-CRP concentrations. Protease inhibitor (PI) use correlated positively with hs-CRP levels, while other HIV-specific parameters (HIV viral load and CD4+ count) were not. Even though hs-CRP collection times were standardized, no adjustment was attempted for the time difference between sample collection and event (or end of follow-up).
Duprez et al [37] sought to identify any association of elevations of three different biomarkers (CRP, interleukin-6 (IL-6), and D-dimer) with increased risk of CVE in a prospective study of 5,098 HIV+ participants enrolled in the SMART (Strategies for Management of Antiretroviral Therapy) trial [41]. The original SMART study enrolled 5,472 HIV+ non-pregnant and non-lactating participants aged > 13 years, with CD4+ counts > 350 cells/mm3, collecting data between January 2002 and January 2006. Participants were followed until July 2017, providing for 18 - 66 months (median: 29 months) follow-up time. Hs-CRP, IL-6 and D-dimer levels were compared between the 252 HIV+ patients who had a CVE (CVD death, non-fatal MI, non-fatal stroke, congestive heart failure (CHF), coronary revascularization, coronary artery disease (CAD) requiring medical treatment, and PAD) and their 4,846 HIV+ counterparts who had no event. The initial univariate analyses adjusted for age and gender. The authors reported a positive association (HR: 2.10, 95% CI: 1.40 - 3.16) between elevated hs-CRP and CVE after adjusting for continuous (viral suppression) versus interrupted use (drug-sparing) ART strategies - the primary independent variable of SMART. This association persisted in multivariate analyses (HR: 1.43, 95% CI: 1.24 - 1.64) after adjusting for the presence of prior CVD, race, smoking status, hypertension treatment, diabetes, hyperlipidemia treatment, total/HDL cholesterol, and other input variables not included in other algorithms (BMI, hepatitis B/C coinfection, and HIV-specific variables including ART naive versus ART exposed, and prior AIDS diagnosis). Any association of CVE with family history of CVD, statin use, HIV duration, or ART regimen or duration of treatment was not reported. Sample collection times for hs-CRP were not standardized, nor was there an adjustment for the differences in time between hs-CRP measurement and index time (event or end of follow-up). Although adjusted, secondary prevention patients (i.e., those with known/established atherosclerosis) were not analyzed as a separate study subgroup.
De Luca et al [38] conducted a case-control analysis of 109 HIV+ patients enrolled in two separate studies based in Rome: the Italian Cohort Naive Antiretrovirals (ICONA) Foundation Cohort [42] and the Catholic University of Sacred Heart (CUSH) clinical database [43]. The CUSH database enrolled male and female HIV+ patients aged 33 - 45 years from 1995 to 2005 to determine the prevalence of drug resistance in individuals failing ART therapy. The ICONA Foundation Cohort enrolled 3,291 HIV+ patients naive to ART between 1997 and 2007 to study the association of ART discontinuation and the time of starting ART to clinical outcomes. Participants from the above two cohorts were included if they were aged 35 - 69 years, had no major CVE prior to initiation of ART, no history of inflammatory disease for at least 3 months prior to CRP sample collection, and no active use of hormonal-based, statin or anti-inflammatory drugs. The authors defined CVE as acute MI, revascularization procedures, stable or unstable angina, analyzing the biomarker both as a categorical (CRP > 3.3 mg/L considered elevated) and as a log-transformed continuous variable. In addition, the analysis was performed using two separate biomarker collection times: reported as late samples (the closest to CV event) and early samples (any available sample prior to the late sample). Thirty-five cases with CVE were compared with 74 event-free controls. Total cholesterol correlated positively with an increased risk of CVE in univariate analysis of late samples, but was adjusted for in multivariate analysis of both late, and combined early and late, CRP samples. Similarly, the duration of exposure to NRTIs, NNRTIs, and PIs correlated positively with an elevated CVE risk in univariate analyses of late samples, and were adjusted for in multivariate analyses of combined samples. Statin use was also adjusted for in this analysis. However, any associations of CVE with the presence of prior CVD, diabetes, hypertension, nicotine dependence, family history of premature ASCVD, ethnicity, or BMI were not reported; although sex did not correlate with CV events, only nine women were included in the study population. Using hs-CRP as a categorical variable, the risk for CVE was significantly higher in those with elevated biomarker in both late (OR: 10.71, 95% CI: 1.03 - 111.0) and combined (OR: 8.00, 95% CI: 1.23 - 51.94) samples after adjustment for the above-mentioned confounding variables. Using hs-CRP as a log-transformed continuous variable, the OR for CVE remained significantly higher in patients with elevated hs-CRP both in late (1.69, 95% CI: 1.09 - 2.64) and combined (1.54, 95% CI: 1.03 - 2.30) samples, after adjustment for the same independent variables. The difference between index time (event for cases, and last follow-up for controls) and hs-CRP collection time was also adjusted for in multivariate analysis.
Studies concluding no association
The retrospective study by Westhorpe et al [39] examined 99 HIV+ patients treated at The Alfred Hospital (Melbourne, Australia) between 1996 and 2003, using a 1:2 case/control design. The outcome of interest was risk of future CVE (defined as MI, an angiogram demonstrating vascular disease, a positive nuclear or exercise stress test, or clinical angina with or without ECG changes) from the comparison of 33 cases with CVE and 66 controls without such events. Concentrations of hs-CRP were analyzed both as a continuous variable (reported as differences in median concentrations (median 3.5 mg/L, IQR 1.6 - 14.4 mg/L for cases; median 2.6 mg/L, IQR 1.2 - 8.3 mg/L for controls; P = 0.20)), and as a categorical variable (defining elevated as a hs-CRP concentration > 5 mg/L) and reported as an OR (1.32, 95% CI: 0.48 - 3.63; P = NS). Thus, the authors did not find a significant association between hs-CRP concentrations and CVE. HIV-specific variables such as viral load, CD4+ count, ART use and ART regimen were matched between event and control patients. Neither existing CVD, more traditional risk factors (such as smoking, hypertension, dyslipidemia, diabetes, BMI, and family history of premature ASCVD), statin use, nor prior AIDS, HIV disease duration or ART duration were analyzed as independent variables. The hs-CRP sample of primary interest was that closest to the event or time of censoring, but adjustment for differences in intervals between sample collection and index time (event or end of follow-up) between the two groups was not reported. The authors noted that this study had only 40% power to predict an outcome difference between hs-CRP-positive and negative patients.
Ford et al [40] conducted a case-control analysis of hs-CRP concentrations and CVE rates in 156 participants from a pool of 1,709 HIV+ patients enrolled in National Institute of Allergy and Infectious Diseases (NIAID)/National Institutes of Health (NIH) intramural clinical protocols between January 1995 and March 2009. This study defined CVE as acute MI, silent MI, acute coronary syndrome, stroke, sudden CV death, coronary revascularization, and/or lower extremity revascularization. Patients with a history of such events prior to their HIV diagnosis, or prior to enrollment in NIAID/NIH studies, were excluded. Cases and controls were matched for time between hs-CRP sample collection and event/index time. The investigators did not find a significant relationship of hs-CRP (collected at two different times) with CVE in comparing 52 cases with CVE to 104 controls without such events. The hs-CRP plasma concentrations were analyzed as the difference in means between the two groups at 4 months (570 ± 41 ng/mL in cases, 570 ± 26 ng/mL in controls; P = 0.60) and 2 years (570 ± 40 ng/mL in cases, 570 ± 26 ng/mL in controls; P = 0.47) prior to the index event. ORs were neither reported in raw univariate analyses nor after adjustment for confounding variables such as dyslipidemia, current smoking status, and family history of premature MI. Any association of CVE with prior CVD was not reported. HIV-specific variables including disease duration, CD4+ count, and ART and PI exposure did not associate with CVE.
| Discussion | ▴Top |
Systemic inflammation has been implicated in CVD pathogenesis at several stages including atherosclerotic plaque formation, plaque destabilization and rupture, and subsequent myocardial injury [18]. Elevated levels of inflammatory biomarkers have been positively associated with CVD risk, fatal CVD events, and overall mortality to varying degrees in the general population [44, 45]. Specifically, elevated hs-CRP concentration appears to predict higher CVD mortality and greater 10-year risk of coronary heart disease (coronary death or MI) in otherwise normal individuals [46, 47]. In another trial, IL-6 and hs-CRP were both more strongly associated with future fatal CVD events than with non-fatal events [48]. A subset of the PROVE-IT TIMI 22 (Pravastatin or Atorvastatin Evaluation and Infection Therapy-Thrombolysis in Myocardial Infarction 22) [49] and of the REVERSAL (Reversal of Atherosclerosis with Aggressive Lipid Lowering) [50] trials found that lower CRP levels are associated with fewer CVE, independent of LDL-cholesterol levels. Measurement of hs-CRP has been suggested to improve CVD risk stratification accuracy among intermediate-risk persons in the JUPITER trial [31]. The addition of hs-CRP in the Reynolds risk score [34, 35] improved CVD risk prediction accuracy, reclassifying 40-50% of women and 20% of men identified as intermediate risk into higher or lower risk categories. To date, this biomarker has not been included in any other standardized risk equations for the general population.
Chronic inflammation and immune activation are consistent findings among HIV+ patients. Hs-CRP has been one of the most studied inflammatory biomarkers for CVE in these patients. Any associations of CVD and cardiovascular mortality with biomarkers of endothelial activation such as sICAM-1, sVCAM-1, VEGF, E-selectin, and coagulation markers such as D-dimer, fibrinogen and tissue factor in this patient cohort remain largely speculative [51]. However, inconsistent findings in studies of hs-CRP association with CVE in HIV+ patients suggest possible confounding with other risk factors. Plasma levels of hs-CRP in fact have been correlated to traditional risk factors in some studies [7, 18, 52-54] suggesting those risk factors either also elevate hs-CRP (whether or not mechanistically) or that their influences on outcomes overlap with hs-CRP. There is however no clear association between ART regimen or treatment duration with hs-CRP levels [17, 41, 44, 55, 56]. No study of HIV+ patients paralleling the JUPITER trial, basing treatment on CRP concentrations, has been attempted to our knowledge.
The studies examined in this review reported conflicting results for association of hs-CRP and CVE in HIV+ patients. Study parameters, outcome definitions, timing between sampling and determination of event status, duration of the assessment period between sampling and event/end of study, and controls for other important independent variables varied widely. The largest and only prospective study in this review [37] reported highly significant associations between three biomarkers (hs-CRP, IL-6 and D-dimer) and coronary events. The composite endpoint, however, included many subjective events (e.g., CHF and CAD requiring drug treatment), with no subgroup analyses by individual endpoint elements. Although family history of CAD was not included as a covariate, the authors adjusted for all other risk factors deemed significant at the time of study, as well as several HIV-specific measures (including ART interruption) and viral hepatitis coinfection history. The design using infected patients in both cohorts excludes the potential bias of investigations comparing HIV+ and HIV- patient outcomes, and this model parallels how hs-CRP might be used as a predictor and treatment guide in infected patients. Median follow-up was 29 months, with some patients followed for as little as 18 months, yielding data far short of the 5 - 10 year window provided by present risk prediction systems; the possibility that events might have occurred later (“catch up”), potentially diluting the strength of biomarker association with CVE, cannot be excluded. Despite these shortcomings, Duprez et al presented the strongest case for hs-CRP association with CVE.
These findings are supported by Triant et al [36]. This investigation had the distinct advantage of measuring only acute MI as an endpoint. The median duration of time from CRP sample event/study closure was 6 years, significantly longer than Duprez. A different model, comparing HIV diagnosis and CRP elevation in a 2 × 2 model found both (independently and when combined) to be associated with future MIs to a high level of statistical significance (P = 0.0122 to < 0.0001) after controlling for several important risk factors, including HIV-specific measures and details of ART exposure; other traditional factors (family history of CAD, smoking rate, and statin use) were reportedly similar between cohorts. No distinction was made between patients with and without previously diagnosed CAD, making it impossible to determine whether this significant association existed specifically in primary prevention patients. Although smaller (< 500 infected patients) than Duprez, the rigor of endpoint selection and covariate assessment strongly supports the association of CRP elevation to future CV events.
The case-control study by DeLuca et al [38] was small (35 infected patients with CVEs) but excluded patients with previous CVEs (i.e., primary prevention). The minimum 5-year data availability from sampling to event/closure per patient was more than Duprez, but the composite endpoint included softer diagnoses (e.g., stable angina) with no separate analyses of endpoint component-determined subgroups. Although matched for smoking and diabetes histories, no adjustments were made for other important covariates such as family history of CVD and hypertension. The significantly higher adjusted OR for elevated CRP (8.00) was likely reduced by these factors.
The two negative retrospective studies by Westhorpe et al [39] and Ford et al [40] were also small (33 and 52 patients with events, respectively). The former was admittedly severely underpowered to find a difference between CRP-determined cohorts, and hence despite matching for HIV-specific factors and analysis for most traditional risk factors, the predictive power of CRP elevation would have needed to be far greater than could reasonably be expected for this study to demonstrate an association. Ford et al limited subjects to primary prevention, and was well-controlled for traditional and HIV-related risk factors. This study however assessed 21 different biomarkers; without a power analysis presented, the degree to which the absence of a demonstrated association can be assured is highly suspect.
Our review has many limitations, including those inherent in database and retrospective analyses. We chose to search only the National Library of Medicine database (possibly missing publications listed only in other databases) and limited our selections to English language journals (that could have missed qualifying non-English journal articles). Our search terms were selected to cast a broad net for potentially applicable papers, but could have resulted in missing some acceptable papers which used more specific key terms. Most of the data (four of five studies) presented were retrospective, and exclusion of specific details (as to why hs-CRP was measured in any individual patient at a specific time, specifics of confounding variables such as details of smoking history, dyslipidemia type, hypertension stage and duration, glycemic control within the included studies, etc.) largely prohibited more thorough comparisons of the studies.
| Conclusion | ▴Top |
Existing CV risk prediction models for the general population do not predict accurately CVE risk in HIV+ patients. The D:A:D model, and models of general population equations recalibrated by incorporating HIV-specific variables or mathematical constants, either under- or over-estimate CVE at various points in the risk spectrum. Although major differences in key components of the included studies were present, our analysis shows the preponderance of the evidence supports the potential value of hs-CRP plasma concentrations as an input variable into studies developing CV risk prediction algorithms for treated HIV+ patients.
Grant Support
No support of any kind was used in the production of this manuscript.
Author Contributions
Both authors contributed substantially to all aspects of manuscript preparation (question definition, literature search, study analyses, writing and referencing) and therefore qualify as authors.
Conflict of Interest
Both authors attest that they have no conflict of interest or relations with industry related to the content of this paper.
| References | ▴Top |
- Bozzette SA, Ake CF, Tam HK, Chang SW, Louis TA. Cardiovascular and cerebrovascular events in patients treated for human immunodeficiency virus infection. N Engl J Med. 2003;348(8):702-710.
doi pubmed - Freiberg MS, Chang CC, Kuller LH, Skanderson M, Lowy E, Kraemer KL, Butt AA, et al. HIV infection and the risk of acute myocardial infarction. JAMA Intern Med. 2013;173(8):614-622.
doi pubmed - Gilbert JM, Fitch KV, Grinspoon SK. HIV-Related Cardiovascular Disease, Statins, and the REPRIEVE Trial. Top Antivir Med. 2015;23(4):146-149.
pubmed - Triant VA, Lee H, Hadigan C, Grinspoon SK. Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. J Clin Endocrinol Metab. 2007;92(7):2506-2512.
doi pubmed - Kaplan RC, Kingsley LA, Sharrett AR, Li X, Lazar J, Tien PC, Mack WJ, et al. Ten-year predicted coronary heart disease risk in HIV-infected men and women. Clin Infect Dis. 2007;45(8):1074-1081.
doi pubmed - Fedele F, Bruno N, Mancone M. Cardiovascular risk factors and HIV disease. AIDS Rev. 2011;13(2):119-129.
pubmed - Samaras K, Gan SK, Peake PW, Carr A, Campbell LV. Proinflammatory markers, insulin sensitivity, and cardiometabolic risk factors in treated HIV infection. Obesity (Silver Spring). 2009;17(1):53-59.
doi pubmed - Schambelan M, Wilson PW, Yarasheski KE, Cade WT, Davila-Roman VG, D'Agostino RB, Sr., Helmy TA, et al. Development of appropriate coronary heart disease risk prediction models in HIV-infected patients. Circulation. 2008;118(2):e48-53.
doi pubmed - Baker J, Ayenew W, Quick H, Hullsiek KH, Tracy R, Henry K, Duprez D, et al. High-density lipoprotein particles and markers of inflammation and thrombotic activity in patients with untreated HIV infection. J Infect Dis. 2010;201(2):285-292.
doi pubmed - Duprez DA, Kuller LH, Tracy R, Otvos J, Cooper DA, Hoy J, Neuhaus J, et al. Lipoprotein particle subclasses, cardiovascular disease and HIV infection. Atherosclerosis. 2009;207(2):524-529.
doi pubmed - Wong G, Trevillyan JM, Fatou B, Cinel M, Weir JM, Hoy JF, Meikle PJ. Plasma lipidomic profiling of treated HIV-positive individuals and the implications for cardiovascular risk prediction. PLoS One. 2014;9(4):e94810.
doi pubmed - Hadigan C, Meigs JB, Wilson PW, D'Agostino RB, Davis B, Basgoz N, Sax PE, et al. Prediction of coronary heart disease risk in HIV-infected patients with fat redistribution. Clin Infect Dis. 2003;36(7):909-916.
doi pubmed - Friis-Moller N, Reiss P, Sabin CA, Weber R, Monforte A, El-Sadr W, Thiebaut R, et al. Class of antiretroviral drugs and the risk of myocardial infarction. N Engl J Med. 2007;356(17):1723-1735.
doi pubmed - Vittecoq D, Escaut L, Chironi G, Teicher E, Monsuez JJ, Andrejak M, Simon A. Coronary heart disease in HIV-infected patients in the highly active antiretroviral treatment era. AIDS. 2003;17(Suppl 1):S70-76.
doi pubmed - Bergersen BM, Sandvik L, Bruun JN, Tonstad S. Elevated Framingham risk score in HIV-positive patients on highly active antiretroviral therapy: results from a Norwegian study of 721 subjects. Eur J Clin Microbiol Infect Dis. 2004;23(8):625-630.
doi pubmed - Calza L, Manfredi R, Pocaterra D, Chiodo F. Risk of premature atherosclerosis and ischemic heart disease associated with HIV infection and antiretroviral therapy. J Infect. 2008;57(1):16-32.
doi pubmed - Baker JV, Neuhaus J, Duprez D, Kuller LH, Tracy R, Belloso WH, De Wit S, et al. Changes in inflammatory and coagulation biomarkers: a randomized comparison of immediate versus deferred antiretroviral therapy in patients with HIV infection. J Acquir Immune Defic Syndr. 2011;56(1):36-43.
doi pubmed - Guimaraes MM, Greco DB, Figueiredo SM, Foscolo RB, Oliveira AR, Jr., Machado LJ. High-sensitivity C-reactive protein levels in HIV-infected patients treated or not with antiretroviral drugs and their correlation with factors related to cardiovascular risk and HIV infection. Atherosclerosis. 2008;201(2):434-439.
doi pubmed - Neuhaus J, Jacobs DR, Jr., Baker JV, Calmy A, Duprez D, La Rosa A, Kuller LH, et al. Markers of inflammation, coagulation, and renal function are elevated in adults with HIV infection. J Infect Dis. 2010;201(12):1788-1795.
doi pubmed - De Lorenzo F, Collot-Teixeira S, Boffito M, Feher M, Gazzard B, McGregor JL. Metabolic-inflammatory changes, and accelerated atherosclerosis in HIV patients: rationale for preventative measures. Curr Med Chem. 2008;15(28):2991-2999.
doi pubmed - Wilson PW, D'Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97(18):1837-1847.
doi pubmed - Conroy RM, Pyorala K, Fitzgerald AP, Sans S, Menotti A, De Backer G, De Bacquer D, et al. Estimation of ten-year risk of fatal cardiovascular disease in Europe: the SCORE project. Eur Heart J. 2003;24(11):987-1003.
doi - D'Agostino RB, Sr., Vasan RS, Pencina MJ, Wolf PA, Cobain M, Massaro JM, Kannel WB. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117(6):743-753.
doi pubmed - Assmann G, Cullen P, Schulte H. Simple scoring scheme for calculating the risk of acute coronary events based on the 10-year follow-up of the prospective cardiovascular Munster (PROCAM) study. Circulation. 2002;105(3):310-315.
doi pubmed - Sritara P, Cheepudomwit S, Chapman N, Woodward M, Kositchaiwat C, Tunlayadechanont S, Sura T, et al. Twelve-year changes in vascular risk factors and their associations with mortality in a cohort of 3499 Thais: the Electricity Generating Authority of Thailand Study. Int J Epidemiol. 2003;32(3):461-468.
doi pubmed - D'Agostino RB, Sr. Cardiovascular risk estimation in 2012: lessons learned and applicability to the HIV population. J Infect Dis. 2012;205(Suppl 3):S362-367.
doi pubmed - Friis-Moller N, Thiebaut R, Reiss P, Weber R, Monforte AD, De Wit S, El-Sadr W, et al. Predicting the risk of cardiovascular disease in HIV-infected patients: the data collection on adverse effects of anti-HIV drugs study. Eur J Cardiovasc Prev Rehabil. 2010;17(5):491-501.
doi pubmed - Markowicz S, Delforge M, Necsoi C, De Wit S. Cardiovascular risk evaluation of HIV-positive patients in a case-control study: comparison of the D:A:D and Framingham equations. J Int AIDS Soc. 2014;17(4 Suppl 3):19515.
doi - Feinstein MJ, Nance RM, Drozd DR, Ning H, Delaney JA, Heckbert SR, Budoff MJ, et al. Assessing and refining myocardial infarction risk estimation among patients with human immunodeficiency virus: a study by the centers for aids research network of integrated clinical systems. JAMA Cardiol. 2017;2(2):155-162.
doi pubmed - Noursadeghi M, Miller RF. Clinical value of C-reactive protein measurements in HIV-positive patients. Int J STD AIDS. 2005;16(6):438-441.
doi pubmed - Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM, Jr., Kastelein JJ, Koenig W, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359(21):2195-2207.
doi pubmed - Ridker PM, Glynn RJ, Hennekens CH. C-reactive protein adds to the predictive value of total and HDL cholesterol in determining risk of first myocardial infarction. Circulation. 1998;97(20):2007-2011.
doi pubmed - Ridker PM, Buring JE, Shih J, Matias M, Hennekens CH. Prospective study of C-reactive protein and the risk of future cardiovascular events among apparently healthy women. Circulation. 1998;98(8):731-733.
doi pubmed - Ridker PM, Paynter NP, Rifai N, Gaziano JM, Cook NR. C-reactive protein and parental history improve global cardiovascular risk prediction: the Reynolds Risk Score for men. Circulation. 2008;118(22):2243-2251, 2244p following 2251.
- Ridker PM, Buring JE, Rifai N, Cook NR. Development and validation of improved algorithms for the assessment of global cardiovascular risk in women: the Reynolds Risk Score. JAMA. 2007;297(6):611-619.
doi pubmed - Triant VA, Meigs JB, Grinspoon SK. Association of C-reactive protein and HIV infection with acute myocardial infarction. J Acquir Immune Defic Syndr. 2009;51(3):268-273.
doi pubmed - Duprez DA, Neuhaus J, Kuller LH, Tracy R, Belloso W, De Wit S, Drummond F, et al. Inflammation, coagulation and cardiovascular disease in HIV-infected individuals. PLoS One. 2012;7(9):e44454.
doi pubmed - De Luca A, de Gaetano Donati K, Colafigli M, Cozzi-Lepri A, De Curtis A, Gori A, Sighinolfi L, et al. The association of high-sensitivity c-reactive protein and other biomarkers with cardiovascular disease in patients treated for HIV: a nested case-control study. BMC Infect Dis. 2013;13:414.
doi pubmed - Westhorpe CL, Schneider HG, Dunne M, Middleton T, Sundararajan V, Spelman T, Carter V, et al. C-reactive protein as a predictor of cardiovascular risk in HIV-infected individuals. Sex Health. 2014;11(6):580-582.
doi pubmed - Ford ES, Greenwald JH, Richterman AG, Rupert A, Dutcher L, Badralmaa Y, Natarajan V, et al. Traditional risk factors and D-dimer predict incident cardiovascular disease events in chronic HIV infection. AIDS. 2010;24(10):1509-1517.
doi pubmed - Strategies for Management of Antiretroviral Therapy Study G, El-Sadr WM, Lundgren J, Neaton JD, Gordin F, Abrams D, Arduino RC, et al. CD4+ count-guided interruption of antiretroviral treatment. N Engl J Med. 2006;355(22):2283-2296.
doi pubmed - Cicconi P, Cozzi-Lepri A, Castagna A, Trecarichi EM, Antinori A, Gatti F, Cassola G, et al. Insights into reasons for discontinuation according to year of starting first regimen of highly active antiretroviral therapy in a cohort of antiretroviral-naive patients. HIV Med. 2010;11(2):104-113.
doi pubmed - Di Giambenedetto S, Bracciale L, Colafigli M, Cattani P, Pinnetti C, Bacarelli A, Prosperi M, et al. Declining prevalence of HIV-1 drug resistance in treatment-failing patients: a clinical cohort study. Antivir Ther. 2007;12(5):835-839.
pubmed - Kuller LH, Tracy R, Belloso W, De Wit S, Drummond F, Lane HC, Ledergerber B, et al. Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med. 2008;5(10):e203.
doi pubmed - Boulware DR, Hullsiek KH, Puronen CE, Rupert A, Baker JV, French MA, Bohjanen PR, et al. Higher levels of CRP, D-dimer, IL-6, and hyaluronic acid before initiation of antiretroviral therapy (ART) are associated with increased risk of AIDS or death. J Infect Dis. 2011;203(11):1637-1646.
doi pubmed - Kuller LH, Tracy RP, Shaten J, Meilahn EN. Relation of C-reactive protein and coronary heart disease in the MRFIT nested case-control study. Multiple Risk Factor Intervention Trial. Am J Epidemiol. 1996;144(6):537-547.
doi pubmed - Cushman M, Arnold AM, Psaty BM, Manolio TA, Kuller LH, Burke GL, Polak JF, et al. C-reactive protein and the 10-year incidence of coronary heart disease in older men and women: the cardiovascular health study. Circulation. 2005;112(1):25-31.
doi pubmed - Sattar N, Murray HM, Welsh P, Blauw GJ, Buckley BM, Cobbe S, de Craen AJ, et al. Are markers of inflammation more strongly associated with risk for fatal than for nonfatal vascular events? PLoS Med. 2009;6(6):e1000099.
doi pubmed - Ridker PM, Cannon CP, Morrow D, Rifai N, Rose LM, McCabe CH, Pfeffer MA, et al. C-reactive protein levels and outcomes after statin therapy. N Engl J Med. 2005;352(1):20-28.
doi pubmed - Nissen SE, Tuzcu EM, Schoenhagen P, Brown BG, Ganz P, Vogel RA, Crowe T, et al. Effect of intensive compared with moderate lipid-lowering therapy on progression of coronary atherosclerosis: a randomized controlled trial. JAMA. 2004;291(9):1071-1080.
doi pubmed - Graham SM, Mwilu R, Liles WC. Clinical utility of biomarkers of endothelial activation and coagulation for prognosis in HIV infection: a systematic review. Virulence. 2013;4(6):564-571.
doi pubmed - Boger MS, Shintani A, Redhage LA, Mitchell V, Haas DW, Morrow JD, Hulgan T. Highly sensitive C-reactive protein, body mass index, and serum lipids in HIV-infected persons receiving antiretroviral therapy: a longitudinal study. J Acquir Immune Defic Syndr. 2009;52(4):480-487.
doi pubmed - Dolan SE, Hadigan C, Killilea KM, Sullivan MP, Hemphill L, Lees RS, Schoenfeld D, et al. Increased cardiovascular disease risk indices in HIV-infected women. J Acquir Immune Defic Syndr. 2005;39(1):44-54.
doi pubmed - Masia M, Bernal E, Padilla S, Graells ML, Jarrin I, Almenar MV, Molina J, et al. The role of C-reactive protein as a marker for cardiovascular risk associated with antiretroviral therapy in HIV-infected patients. Atherosclerosis. 2007;195(1):167-171.
doi pubmed - Shikuma CM, Ribaudo HJ, Zheng Y, Gulick RM, Meyer WA, Tashima KT, Bastow B, et al. Change in high-sensitivity c-reactive protein levels following initiation of efavirenz-based antiretroviral regimens in HIV-infected individuals. AIDS Res Hum Retroviruses. 2011;27(5):461-468.
doi pubmed - Wada NI, Jacobson LP, Margolick JB, Breen EC, Macatangay B, Penugonda S, Martinez-Maza O, et al. The effect of HAART-induced HIV suppression on circulating markers of inflammation and immune activation. AIDS. 2015;29(4):463-471.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Medicine Research is published by Elmer Press Inc.











