| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website http://www.jocmr.org |
Original Article
Volume 9, Number 3, March 2017, pages 193-199
Efficacy and Safety of Vildagliptin as an Add-On Therapy in Inadequately Controlled Type 2 Diabetes Patients Treated With Basal Insulin
Daisuke Saitoa, Akio Kanazawaa, b, e, Nayumi Shigiharaa, Fumihiko Satoa, Toyoyoshi Uchidaa, Junko Satoa, Hiromasa Gotoa, Takeshi Miyatsukaa, Fuki Ikedaa, Takeshi Ogiharaa, Chie Ohmuraa, Hirotaka Watadaa, b, c, d
aDepartment of Metabolism & Endocrinology, Juntendo University Graduate School of Medicine, Tokyo, Japan
bCenter for Therapeutic Innovations in Diabetes, Juntendo University Graduate School of Medicine, Tokyo, Japan
cCenter for Identification of Diabetic Therapeutic Targets, Juntendo University Graduate School of Medicine, Tokyo, Japan
dSportology Center, Juntendo University Graduate School of Medicine, Tokyo, Japan
eCorresponding Author: Akio Kanazawa, Department of Metabolism & Endocrinology, Juntendo University Graduate School of Medicine, 2-1-1 Hongo, Bunkyou-ku, Tokyo 113-8421, Japan
Manuscript accepted for publication December 21, 2016
Short title: Efficacy of Vildagliptin as an Add-On Therapy
doi: https://doi.org/10.14740/jocmr2874w
| Abstract | ▴Top |
Background: The aim of this study was to investigate the efficacy and safety of vildagliptin as an add-on therapy for patients with type 2 diabetes mellitus inadequately controlled with basal insulin.
Methods: Twenty-four patients treated with basal insulin and oral anti-diabetes drugs were randomly allocated into two groups: the control group (did not receive any add-on drugs) and vildagliptin group (received vildagliptin 100 mg/day for 6 months). The primary outcome was changes in hemoglobin A1c (HbA1c) from baseline to end of study.
Results: Treatment with vildagliptin significantly reduced HbA1c from 8.1±0.7% at baseline to 7.1±0.7% (P < 0.01), while there was no significant change of HbA1c in the control group. Vildagliptin group showed significant reduction of HbA1c compared with control group (-1.0±0.3% vs. 0.2±0.8%, P < 0.01). In addition, vildagliptin group showed a significant increase in 1,5-anhydroglucitol compared with the control group (4.5 ± 3.4 vs. 0.5 ± 4.1 μg/mL, P < 0.05). Mild hypoglycemia was reported in one patient of the vildagliptin group and two patients of the control group.
Conclusion: Vildagliptin improved glycemic control without increasing hypoglycemia in Japanese type 2 diabetes inadequately controlled with basal insulin treatment and other oral anti-diabetes drugs. This study was registered with UMIN (University Hospital Medical Information Network ID#000010849).
Keywords: Basal insulin; DPP-4 inhibitor; 1,5-anhydroglucitol
| Introduction | ▴Top |
The main goals of treatment of diabetes mellitus are to prevent diabetic complications and maintain good quality of life. For this purpose, intensive treatment using various oral anti-diabetes drugs (OADs) is often required in daily practice. However, due to the progressive nature of type 2 diabetes mellitus (T2DM), many T2DM patients eventually require insulin therapy to achieve better glycemic control [1]. However, patients are often reluctant to be treated with insulin for several reasons, such as increased risk of hypoglycemia, fear or inconvenience to “injection”, and concerns of complex insulin regimens. For similar reasons, physicians are also reluctant to introduce insulin therapy. It is no exaggeration to say that these factors have led to delayed insulin initiation at least in Japan [2]. In contrast, treatment regimens that include the addition of long-acting basal insulin injection to ongoing treatment with OADs have been widely adopted for patients with T2DM [3]. The simple and once-daily injection is expected to reduce the psychological burden of insulin treatment and is clinically effective (more than 1.0% reduction from the baseline) [4]. However, among the patients treated with basal insulin and OADs, 73.1% patients did not achieve target hemoglobin A1c (HbA1c) level (< 7.0%) [3]. This result clearly suggests the need for additional treatment.
Dipeptidyl peptidase-4 (DPP-4) inhibitors increase the serum concentrations of glucagon-like peptide-1, which promotes glucose-response insulin secretion and inhibits glucagon secretion from α cells [5]. Accordingly, these drugs can decrease both fasting and postprandial glucose levels [6] with low risk of hypoglycemia and without body weight gain. Additionally, the glucose-lowering effect is known to be greater in Asians than Caucasians [7]. Considering the favorable effects of these drugs, DPP-4 inhibitors are frequently added in Japan to treatment regimens that contain basal insulin as the next option for better glycemic control. Furthermore, as vildagliptin strongly inhibits DPP-4 activity by covalently binding to DPP-4 [8], this drug might show superior glucose-lowering effects in uncontrolled patients treated with basal insulin. However, there have been only a few randomized control studies that investigated the efficacy and safety of DPP-4 inhibitors as an add-on drug in patients treated with basal insulin. Therefore, we conducted a 6-month randomized control trial to investigate the efficacy and safety of add-on therapy of vildagliptin. This is the first randomized controlled trial to investigate the effect of vildagliptin, a DPP-4 inhibitor, in Japanese patients with T2DM who were inadequately controlled with basal insulin alone and OADs.
| Materials and Methods | ▴Top |
Subjects
T2DM patients were recruited from the Outpatient Clinic of Juntendo University Hospital, Juntendo Shizuoka Hospital, Tokyo Joto Hospital and International Goodwill Hospital, between May 2013 and April 2015. The following inclusion criteria were applied at study registration: 1) T2DM patients aged > 20 but < 80 years; 2) T2DM patients on combination therapy of OADs, excluding DPP-4 inhibitors, and basal insulin alone; and 3) T2DM patients with HbA1c (National Glycohemoglobin Standardization Program: NGSP) ≥ 7.0% despite treatment of targeting fasting blood glucose (FBG) below 110 mg/dL by titration of basal insulin. The selected T2DM patients were excluded from the study if any of the following conditions was diagnosed at registration: 1) proliferative retinopathy, 2) severe neuropathy, 3) serious kidney disease (serum creatinine level > 2.0 mg/dL), 4) serious liver disease, 5) acute heart failure, 6) pregnancy, 7) serious infectious disease, 8) trauma injury, 9) pituitary insufficiency, and 10) not suitable for the study. The subjects were screened consecutively, and patients that met the above eligibility criteria were asked to participate in the present study.
The study protocol was approved by the Human Ethics Committee of Juntendo University, and written informed consent was obtained from each patient before enrollment in the study.
Study design
Determination of sample size
We performed an open-label, two-arm, randomized controlled study. Vildagliptin is known to reduce HbA1c by 0.6% with 0.1% standard deviation (SD) in T2DM patients on insulin treatment [9]. In our study, we adopt 0.5% SD considering data variability. With a two-sided α level of 5% and power (1 - β) of 90%, at least 24 patients (12 patients in each group) were required to confirm the superiority of vildagliptin in reducing HbA1c. Therefore, we recruited 24 patients who were assigned randomly to either the control group (12 patients) or the vildagliptin group (12 patients) for 6 months. Randomization was achieved by the minimization and biased coin method (Soiken, Inc., Osaka, Japan). The primary endpoint was a change in HbA1c level at the end of the study from the baseline. The secondary endpoint was daily glucose profile by self-monitoring of blood glucose (SMBG). A six-point SMBG (before and 2 h after the meal) was performed at the baseline and 6 months after the beginning of the study using glucometer (Glutest Neo Super®, Sanwa Kagaku Kenkyusho, Japan). Otherwise, patients were instructed to measure FBG daily and other blood glucose whenever necessary. The frequency and severity of hypoglycemia, if any, were evaluated during the study period. Severe hypoglycemia was defined as low blood glucose level that required assistance from another person to treat.
Add-on vildagliptin
In the vildagliptin group, vildagliptin 100 mg/day was added to the current therapy of basal insulin alone. Both groups were observed for 6 months and asked to visit the hospital every month. In both groups, the change in dosage of the current OADs or the addition of new drugs was not allowed during the 6-month study period. However, the dose of basal insulin was adjusted to achieve the target FBG below 110 mg/dL (110 ≤ FBG < 140 mg/dL: 1 unit increase of basal insulin; FBG ≥ 140 mg/dL: 2 units increase of basal insulin), and decrease of basal insulin was judged by each attending physician in order to avoid hypoglycemia.
Biochemical tests
Blood samples were obtained at overnight fasting. C-peptide index was calculated as follows: serum C-peptide (ng/mL)/plasma glucose (mg/dL) × 100. Serum total cholesterol (T-CHO), high-density lipoprotein cholesterol (HDL-C), triglyceride (TG), FBG, and HbA1c were measured with standard techniques. 1,5-anhydroglucitol and urinary albumin were measured by the enzymatic method and immune nephelometric method, respectively. These tests were performed at baseline and after 6-month treatment with vildagliptin.
Statistical analysis
Data were expressed as mean ± SD for normally distributed data and median (interquartile range) for data with skewed distribution. The Mann-Whitney U test and unpaired t-test test were used for data analysis. A P value < 0.05 denoted the presence of a statistically significant difference. All statistical analyses were conducted using StatView statistical software package, version 5.0 (SAS Institute Inc., Cary, NC).
| Results | ▴Top |
Baseline characteristics of subjects
Of the 24 patients recruited in the study, 12 patients were assigned to the control group and 12 to the vildagliptin group. As shown in Figure 1, all patients completed the 6-month study. The baseline characteristics of those patients who completed the study are summarized in Table 1. At baseline, HbA1c levels in the control and vildagliptin group were 7.7±0.6% and 8.1±0.7%, respectively, and the level was not significantly different between the two groups. The body mass index (BMI) and dosage of basal insulin in the control and vildagliptin groups were 24.8 ± 5.3 and 25.6 ± 5.4 kg/m2, and 9.4 ± 2.3 and 10.4 ± 6.4 unit/day, respectively. Again, there were no significant differences in these parameters between the two groups. The data of these baseline characteristics matched those of Japanese patients with T2DM who participated in the ALOHA-2 study [3].
 Click for large image | Figure 1. Flow diagram of patient recruitment. Twenty-four patients were randomly allocated to either the control group or the vildagliptin group. All patients were followed up for 6 months. |
 Click to view | Table 1. Characteristics of Subjects at Baseline |
Effects of add-on therapy of vildagliptin on HbA1c and SMBG
Figure 2 shows the serial changes in HbA1c for both groups. At 2, 3, 5 and 6 months after the start of the study, HbA1c was significantly lower, relative to the baseline, in the vildagliptin group, but not in the control group. The change in HbA1c from baseline to 6 months after the beginning of the study was significantly higher (-1.0±0.3%) in the vildagliptin group than the control group (0.21±0.81%, P < 0.01) (Fig. 2 and Table 2).
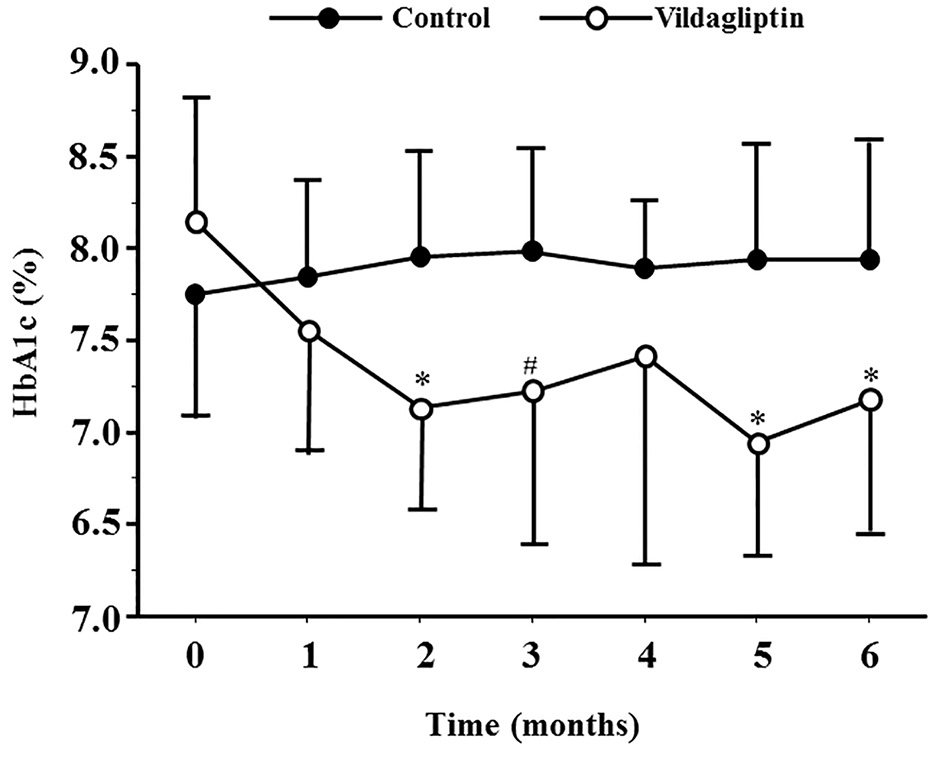 Click for large image | Figure 2. HbA1c levels during the study period in the control and vildagliptin groups. *P < 0.01, #P < 0.05, compared with the control group. |
 Click to view | Table 2. Changes in Clinical Parameters Relative to the Baseline |
Figure 3 shows the results of SMBG recorded at baseline and study end. In the vildagliptin group, blood glucose levels at 2 h after breakfast, before dinner and 2 h after dinner at the end of the 6-month study were significantly lower than those measured at baseline (215.0 ± 72.3 vs. 163.4 ± 48.3 mg/dL, P < 0.05, 154.6 ± 56.8 vs. 102.7 ± 21.4 mg/dL, P < 0.05 and 207.9 ± 52.4 vs. 163.4 ± 39.9 mg/dL, P < 0.05, respectively). In contrast, blood glucose levels showed no such significant differences in the control group.
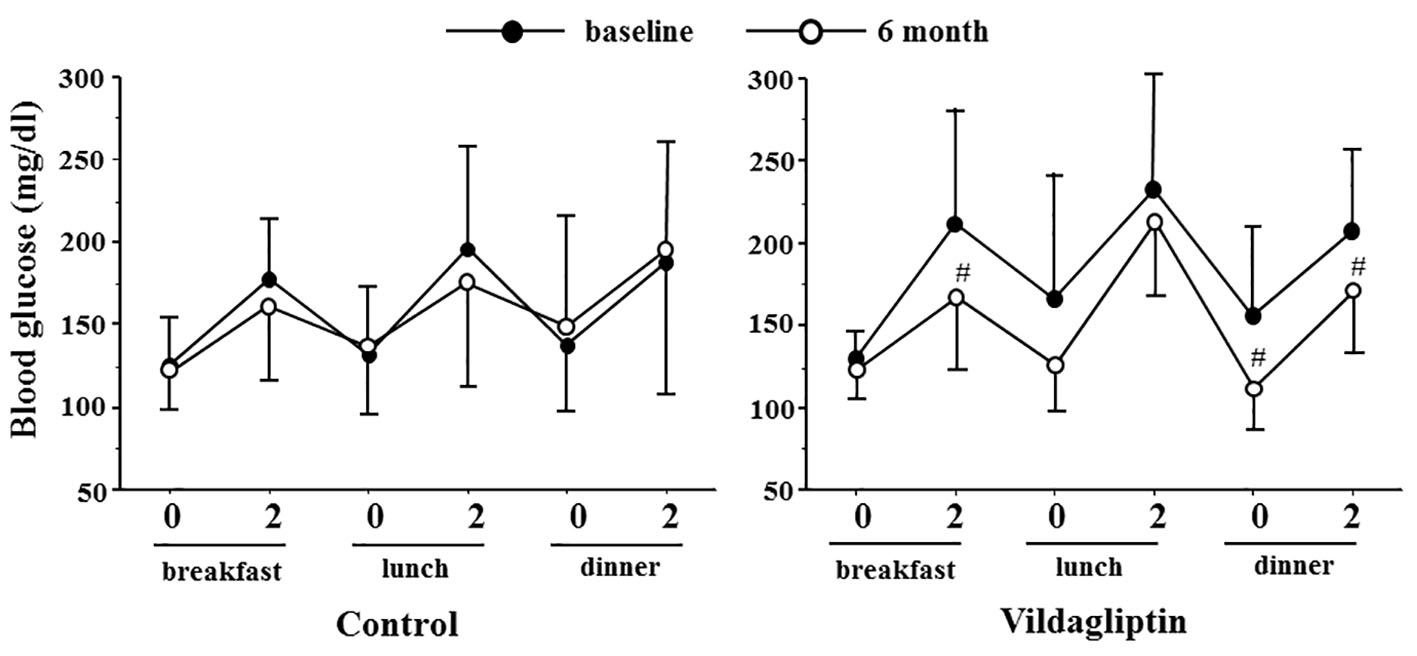 Click for large image | Figure 3. Blood glucose profile in the control and vildagliptin groups measured before (0 M) and after 6 months (6 M). 0 and 2: blood glucose level before and 2 h after the indicated meal. Vildagliptin significantly improved blood glucose levels 2 h after breakfast and dinner, and blood glucose levels before dinner compared with the baseline. #P < 0.05, compared with the baseline. |
Effects of add-on therapy of vildagliptin on 1,5-anhydroglucitol and other parameters
In addition to HbA1c, the change in 1,5-anhydroglucitol from baseline to the end of the study was also higher in the vildagliptin group compared to the control group (4.5 ± 3.4 vs. 0.5 ± 4.1 μg/mL, P = 0.03). On the other hand, changes in BMI, FBG, C-peptide index, urinary albumin excretion rate and insulin unit were comparable between the two groups (Table 2). With regard to the secondary outcome, only one patient of the vildagliptin group and two patients of the control group developed mild hypoglycemia, and this was limited to a single episode in each of these patients. Importantly, no severe hypoglycemia that required assistance by other person and no serious adverse events were reported in both groups during the study period.
| Discussion | ▴Top |
The important finding of this study is that add-on 100 mg/day vildagliptin in patients with inadequately controlled T2DM by basal insulin and OADs resulted in significant improvement of glycemic control without increased hypoglycemia. Vildagliptin treatment in our study reduced HbA1c by about 1.0% from the baseline. This improvement is greater compared to previous studies that investigated the effect of the combination of vildagliptin and insulin [9, 10] in Western countries but was similar to that reported in a previous Japanese study [11]. The baseline HbA1c reported in previous studies in Western countries averaged from 8.4% [9] to 8.8% [10], which are higher than the baseline HbA1c levels in our patients. A greater fall in HbA1c could result from treatment of patients with higher baseline HbA1c levels. For this reason, the reduction in HbA1c recorded in our study was considered high despite the lower baseline HbA1c levels. Considering the greater glucose-lowering effect of DPP-4 inhibitors in Asians compared with Caucasians [7], the high efficacy of DPP-4 inhibitors observed in this study is reasonable.
Other reasons for effectiveness of vildagliptin in this study include preserved β-cell function, as reflected by C-peptide index. Previous studies reported that C-peptide index could predict the importance and need of insulin therapy [12, 13]. Furthermore, Kozawa et al [14] reported that the mean value of C-peptide index required to maintain good glycemic control after switching from insulin therapy to DPP-4 inhibitors in their in-patient study was 1.5 ± 0.5 ng/mL. Therefore, β-cell function of the vildagliptin group (mean C-peptide index: 1.38 ± 0.76 ng/mL) was considered to be preserved and sensitive to treatment with vildagliptin.
In this study, we investigated the daily glycemic profile by SMBG, and reported that the vildagliptin add-on to basal insulin improved mainly postprandial hyperglycemia, although FBG levels were not different before and after the add-on treatment with vildagliptin. Based on these findings, it can be concluded that the reduction in HbA1c was due to improvement in postprandial hyperglycemia. Indeed, we also observed an increase in 1,5-anhydroglucitol after vildagliptin. Glucose variability is reported to be one of the risk factors for cardiovascular diseases [15, 16] and cognitive dysfunction [17, 18]. Therefore, the combination therapy of vildagliptin and basal insulin is a beneficial option for the treatment of elderly patients with cardiovascular diseases who do not accept intensive insulin therapy and need to avoid hypoglycemia.
Limitations
Our study has the following limitations; the number of study subjects was too small to confirm the effects of vildagliptin on β-cell function. Previous report showed that DPP-4 inhibitor increased C-peptide index, and patients with increased C-peptide index were responders of DPP-4 inhibitor [19]. In our study, the change in C-peptide index after vildagliptin treatment tended to be higher than the control. Therefore, a larger sample size is needed in the future to address this issue.
Conclusions
Our study showed that 100 mg/day vildagliptin add-on to basal insulin alone improved both glycemic control and glucose fluctuation in Japanese patients with T2DM without increased hypoglycemia.
Acknowledgments
We thank all staff of Juntendo University Hospital, Juntendo Shizuoka Hospital, Tokyo Joto Hospital and International Goodwill Hospital for the excellent assistance.
Competing Interests
AK has received lecture fees from Kissei Pharma, Sanofi and Takeda Pharmaceutical Co. HW has received lecture fees from Asteras, Astrazeneca, Boehringer Ingelheim, Daiichi Sankyo Inc., Eli Lilly and Company, Kissei Pharmaceutical Co., Kowa Pharmaceutical Co., Kyowa Hakko Kirin Co., MSD, Novartis Pharmaceuticals, Novo Nordisk Pharma, Ono Pharmaceutical Co., Mitsubishi Tanabe Pharma, Sanofi-Aventis, Sanwakagaku Kenkyusho, and Takeda Pharmaceutical Co. and research funds from Asteras, Astrazeneca, Bristol-Myers Squibb, Boehringer Ingelheim, Daiichi Sankyo Inc., Dainippon Sumitomo Pharma, Eli Lilly, Johnson and Johnson, Kissei Pharmaceutical Co., Kowa Pharmaceutical Co., Kyowa Hakko Kirin Co., MSD, Mitsubishi Tanabe Pharma, Mochida Pharmaceutical Co., Novartis Pharmaceuticals, Novo Nordisk Pharma, Pfizer, Sanwakagaku Kenkyusho, Sanofi, and Takeda Pharmaceutical Co. All other authors report no conflict of interest.
Grant Support
None.
| References | ▴Top |
- Home P, Riddle M, Cefalu WT, Bailey CJ, Bretzel RG, Del Prato S, Leroith D, et al. Insulin therapy in people with type 2 diabetes: opportunities and challenges? Diabetes Care. 2014;37(6):1499-1508.
doi pubmed - Ishii H, Iwamoto Y, Tajima N. An exploration of barriers to insulin initiation for physicians in Japan: findings from the Diabetes Attitudes, Wishes And Needs (DAWN) JAPAN study. PLoS One. 2012;7(6):e36361.
doi pubmed - Tsukube S, Ikeda Y, Kadowaki T, Odawara M. Improved Treatment Satisfaction and Self-reported Health Status after Introduction of Basal-Supported Oral Therapy Using Insulin Glargine in Patients with Type 2 Diabetes: Sub-Analysis of ALOHA2 Study. Diabetes Ther. 2015;6(2):153-171.
doi pubmed - Odawara M, Kadowaki T, Naito Y. Effectiveness and safety of basal supported oral therapy with insulin glargine, in Japanese insulin-naive, type 2 diabetes patients, with or without microvascular complications: subanalysis of the observational, non-interventional, 24-week follow-up Add-on Lantus(R) to Oral Hypoglycemic Agents (ALOHA) study. J Diabetes Complications. 2015;29(1):127-133.
doi pubmed - Omar B, Ahren B. Pleiotropic mechanisms for the glucose-lowering action of DPP-4 inhibitors. Diabetes. 2014;63(7):2196-2202.
doi pubmed - Xu L, Man CD, Charbonnel B, Meninger G, Davies MJ, Williams-Herman D, Cobelli C, et al. Effect of sitagliptin, a dipeptidyl peptidase-4 inhibitor, on beta-cell function in patients with type 2 diabetes: a model-based approach. Diabetes Obes Metab. 2008;10(12):1212-1220.
doi - Kim YG, Hahn S, Oh TJ, Kwak SH, Park KS, Cho YM. Differences in the glucose-lowering efficacy of dipeptidyl peptidase-4 inhibitors between Asians and non-Asians: a systematic review and meta-analysis. Diabetologia. 2013;56(4):696-708.
doi pubmed - Ahren B, Schweizer A, Dejager S, Villhauer EB, Dunning BE, Foley JE. Mechanisms of action of the dipeptidyl peptidase-4 inhibitor vildagliptin in humans. Diabetes Obes Metab. 2011;13(9):775-783.
doi pubmed - Fonseca V, Schweizer A, Albrecht D, Baron MA, Chang I, Dejager S. Addition of vildagliptin to insulin improves glycaemic control in type 2 diabetes. Diabetologia. 2007;50(6):1148-1155.
doi pubmed - Kothny W, Foley J, Kozlovski P, Shao Q, Gallwitz B, Lukashevich V. Improved glycaemic control with vildagliptin added to insulin, with or without metformin, in patients with type 2 diabetes mellitus. Diabetes Obes Metab. 2013;15(3):252-257.
doi pubmed - Hirose T, Bailey CP. The "universal" cardiovascular cannula. A tapered corrugated plastic catheter for cannulation in extracorporeal circulation. J Thorac Cardiovasc Surg. 1962;43:559-560.
pubmed - Goto A, Takaichi M, Kishimoto M, Takahashi Y, Kajio H, Shimbo T, Noda M. Body mass index, fasting plasma glucose levels, and C-peptide levels as predictors of the future insulin use in Japanese type 2 diabetic patients. Endocr J. 2010;57(3):237-244.
doi pubmed - Funakoshi S, Fujimoto S, Hamasaki A, Fujiwara H, Fujita Y, Ikeda K, Takahara S, et al. Utility of indices using C-peptide levels for indication of insulin therapy to achieve good glycemic control in Japanese patients with type 2 diabetes. J Diabetes Investig. 2011;2(4):297-303.
doi pubmed - Kozawa J, Kitamura T, Nishizawa H, Yasuda T, Maeda N, Otsuki M, Okita K, et al. Dipeptidyl peptidase-4 inhibitors are effective in Japanese type 2 diabetic patients with sustained endogenous insulin-secreting capacity, a higher body mass index and insulin resistance. J Diabetes Investig. 2013;4(2):190-194.
doi pubmed - Su G, Mi SH, Tao H, Li Z, Yang HX, Zheng H, Zhou Y, et al. Impact of admission glycemic variability, glucose, and glycosylated hemoglobin on major adverse cardiac events after acute myocardial infarction. Diabetes Care. 2013;36(4):1026-1032.
doi pubmed - Kataoka S, Gohbara M, Iwahashi N, Sakamaki K, Nakachi T, Akiyama E, Maejima N, et al. Glycemic Variability on Continuous Glucose Monitoring System Predicts Rapid Progression of Non-Culprit Lesions in Patients With Acute Coronary Syndrome. Circ J. 2015;79(10):2246-2254.
doi pubmed - Rizzo MR, Marfella R, Barbieri M, Boccardi V, Vestini F, Lettieri B, Canonico S, et al. Relationships between daily acute glucose fluctuations and cognitive performance among aged type 2 diabetic patients. Diabetes Care. 2010;33(10):2169-2174.
doi pubmed - Kim C, Sohn JH, Jang MU, Kim SH, Choi MG, Ryu OH, Lee S, et al. Association between Visit-to-Visit Glucose Variability and Cognitive Function in Aged Type 2 Diabetic Patients: A Cross-Sectional Study. PLoS One. 2015;10(7):e0132118.
doi pubmed - Nishimura T, Meguro S, Sekioka R, Tanaka K, Saisho Y, Irie J, Tanaka M, et al. C-peptide immunoreactivity index is associated with improvement of HbA1c: 2-Year follow-up of sitagliptin use in patients with type 2 diabetes. Diabetes Res Clin Pract. 2015;108(3):441-447.
doi pubmed
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Medicine Research is published by Elmer Press Inc.











