| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website http://www.jocmr.org |
Original Article
Volume 8, Number 12, December 2016, pages 888-892
Efficacy and Safety of a Single-Pill Fixed-Dose Combination of Azilsartan and Amlodipine
Kota Motozatoa, b, Shin-ichiro Miuraa, c, d, Yuhei Shigaa, Takaaki Kusumotob, Keijiro Sakua, c
aDepartment of Cardiology, Fukuoka University School of Medicine, Fukuoka, Japan
bIzumi General Medical Center, Kagoshima, Japan
cDepartment of Molecular Cardiovascular Therapeutics, Fukuoka University School of Medicine, Fukuoka, Japan
dCorresponding Author: Shin-ichiro Miura, Department of Cardiology, Fukuoka University School of Medicine, 7-45-1 Nanakuma, Jonan-ku, Fukuoka 814-0180, Japan
Manuscript accepted for publication September 22, 2016
Short title: Efficacy of Azilsartan and Amlodipine
doi: http://dx.doi.org/10.14740/jocmr2768w
| Abstract | ▴Top |
Background: Guidelines for the management of hypertension recommend the use of drugs with different mechanisms of action in antihypertensive regimens that include single-pill fixed-dose combinations of medications. There is some controversy regarding which single-pill fixed-dose combinations of angiotensin II type 1 receptor blockers (ARBs) and calcium channel blockers (CCBs) are effective at reducing blood pressure (BP).
Methods: Forty hypertensive patients who were receiving a single-pill fixed-dose combination of valsartan 80 mg/day and amlodipine 5 mg/day or irbesartan 100 mg/day and amlodipine 5 mg/day were enrolled. They were randomly divided into two treatment groups, a group that changed to a single-pill fixed-dose combination of azilsartan 20 mg/day and amlodipine 5 mg/day (changeover group) and a group that continued to receive valsartan 80 mg/day and amlodipine 5 mg/day or irbesartan 100 mg/day and amlodipine 5 mg/day (control group), and treated for 16 weeks.
Results: There were no significant differences in systolic blood pressure (SBP), diastolic blood pressure (DBP) or pulse rate (PR) at 16 weeks between the control and changeover groups. In addition, there were no significant changes in biochemical parameters throughout the study period in both groups.
Conclusion: The ability of a single-pill fixed-dose combination of azilsartan and amlodipine to reduce BP may be comparable to that of a combination of valsartan and amlodipine or irbesartan and amlodipine.
Keywords: Angiotensin II type 1; Receptor; Blockers; Calcium channel; Blood pressure
| Introduction | ▴Top |
Although optimal blood pressure (BP) control is associated with remarkable clinical benefits with regard to cardiovascular and renal protection, many patients still show higher BP. Various guidelines recommend different combinations of angiotensin II type 1 receptor blockers (ARBs) and calcium channel blockers (CCBs) [1, 2]. Most patients with hypertension (HTN) require two or more drugs to achieve their target BP [3]. Recently, many kinds of single-pill fixed-dose combinations of ARBs and CCBs have become available for clinical use in Japan, and have been shown to be helpful for controlling BP [4]. However, there is still some controversy regarding which single-pill fixed-dose combinations of ARBs and CCBs are effective for all types of HTN.
Azilsartan is the newest ARB to be approved for clinical use in Japan, and has a significant BP-lowering effect. Azilsartan medoxomil and azilsartan have been reported to have greater antihypertensive effects than other ARBs [5-8]. Azilsartan has been shown to bind tightly to and dissociate slowly from AT1 receptors [9]. We hypothesized that the depressor effect of azilsartan with CCB may be superior to those of other ARBs with CCB in patients with HTN. Therefore, in this study, we compared the efficacy and safely of a single-pill fixed-dose combination of azilsartan and amlodipine to those of combinations of irbesartan or valsartan and amlodipine.
| Methods | ▴Top |
Study design
Forty hypertensive patients in whom BP was controlled with the use of a single-pill fixed-dose combination of valsartan 80 mg/day and amlodipine 5 mg/day (Exforge®) or irbesartan 100 mg/day and amlodipine 5 mg/day (Aimix®LD) were enrolled. They were randomly divided into two treatment groups: a group that changed to a single-pill fixed-dose combination of azilsartan 20 mg/day and amlodipine 5 mg/day (Zacras®, changeover group) and a group that continued to receive valsartan 80 mg/day and amlodipine 5 mg/day or irbesartan 100 mg/day and amlodipine 5 mg/day (control group). One patient withdrew during the study period because she was admitted to the hospital with non-cardiovascular disease. Therefore, we finally analyzed 39 hypertensive patients (20 and 19 in the control and changeover groups, respectively).
Office systolic blood pressure (SBP), diastolic blood pressure (DBP) and pulse rate (PR) measurements were obtained at 0, 4, 8, 12 and 16 weeks. The target BP followed the Japanese Society of Hypertension Guidelines for the Management of Hypertension 2009 (JSH2009) [1]. We excluded patients with liver dysfunction, renal dysfunction (defined as a serum creatinine (Cr) level of more than 2.0 mg/dL), pregnancy, or a history of allergy to the study drugs. The protocol in this study was approved by the ethics committee of Fukuoka University Hospital (#14-5-03) and registered under UMIN000016251, and all subjects gave their written informed consent to participate.
Evaluation of clinical parameters
BP was determined as the mean of two measurements obtained in an office setting by the conventional cuff method using a mercury sphygmomanometer after at least 5 min of rest. We analyzed the levels of biochemical parameters in blood at 0, 8 and 16 weeks. Blood samples were collected in the morning after the patients had fasted overnight. Data regarding serum levels of biochemical parameters, such as high-density lipoprotein-cholesterol (HDL-C), low-density lipoprotein-cholesterol (LDL-C), triglycerides (TG), uric acid (UA), fasting plasma glucose and hemoglobin A1c, sodium, potassium, Cr and brain natriuretic peptide were collected in all patients. Body mass index (BMI) was calculated as weight (kg)/height (m)2.
The characteristics of the patients, with regard to history of HTN, dyslipidemia (DL), diabetes mellitus (DM), hyperuricemia (HU), chronic kidney disease (CKD) and medication use, were obtained from medical records. Patients who had a current SBP/DBP ≥ 140/90 mm Hg or who were receiving antihypertensive therapy were considered to have HTN. Patients with LDL-C ≥ 140 mg/dL, TG ≥ 150 mg/dL, and/or HDL-C < 40 mg/dL, or who were receiving lipid-lowering therapy, were considered to have DL. DM was defined using the Japan Diabetes Society criteria or the use of a glucose-lowering drug. HU was defined as a serum UA level of ≥ 7.0 mg/dL or the use of uric acid-lowering drugs.
Statistical analysis
Statistical analysis was performed using the Stat View statistical software package (Stat View 5; SAS Institute Inc., Cary, NC, USA) at Fukuoka University (Fukuoka, Japan). Data were shown as the mean ± standard deviation (SD). Categorical variables and continuous variables were compared between groups using a Chi-square analysis and unpaired t-test, respectively. Changes in SBP, DBP, PR, and clinical parameters following therapy were analyzed by the paired t-test. A value of P < 0.05 was considered significant.
| Results | ▴Top |
Patient characteristics
Table 1 shows the characteristics of the 39 patients containing 17 (44%) males. The changeover and control groups consisted of 19 and 20 patients, respectively. DM, DL and HU were observed in 33%, 77% and 28% of all subjects, respectively. The mean age was 73 ± 12 years, and BMI was 25 ± 4 kg/m2. The incidences of several coronary risk factors such as gender, BMI, smoking, DM and DL were similar in the control and changeover groups. There was no significant difference in the use of medications such as β-blocker, α-blocker and diuretic between the groups. We did not change these medications throughout the study period.
 Click to view | Table 1. Baseline Characteristics in All Patients, the Control and Changeover Groups (Mean ± SD) |
Time courses of SBP, DBP and PR in the control and changeover groups
The time courses of SBP, DBP and PR in the control and changeover groups are shown in Figure 1. There was no difference in SBP and DBP at 0 week between the groups. In the changeover group, there was no difference in SBP, DBP or PR throughout the study period. Although DBPs in the control group were significantly decreased at 4, 8 and 12 weeks, there was no difference between DBP at 16 weeks and that at 0 weeks.
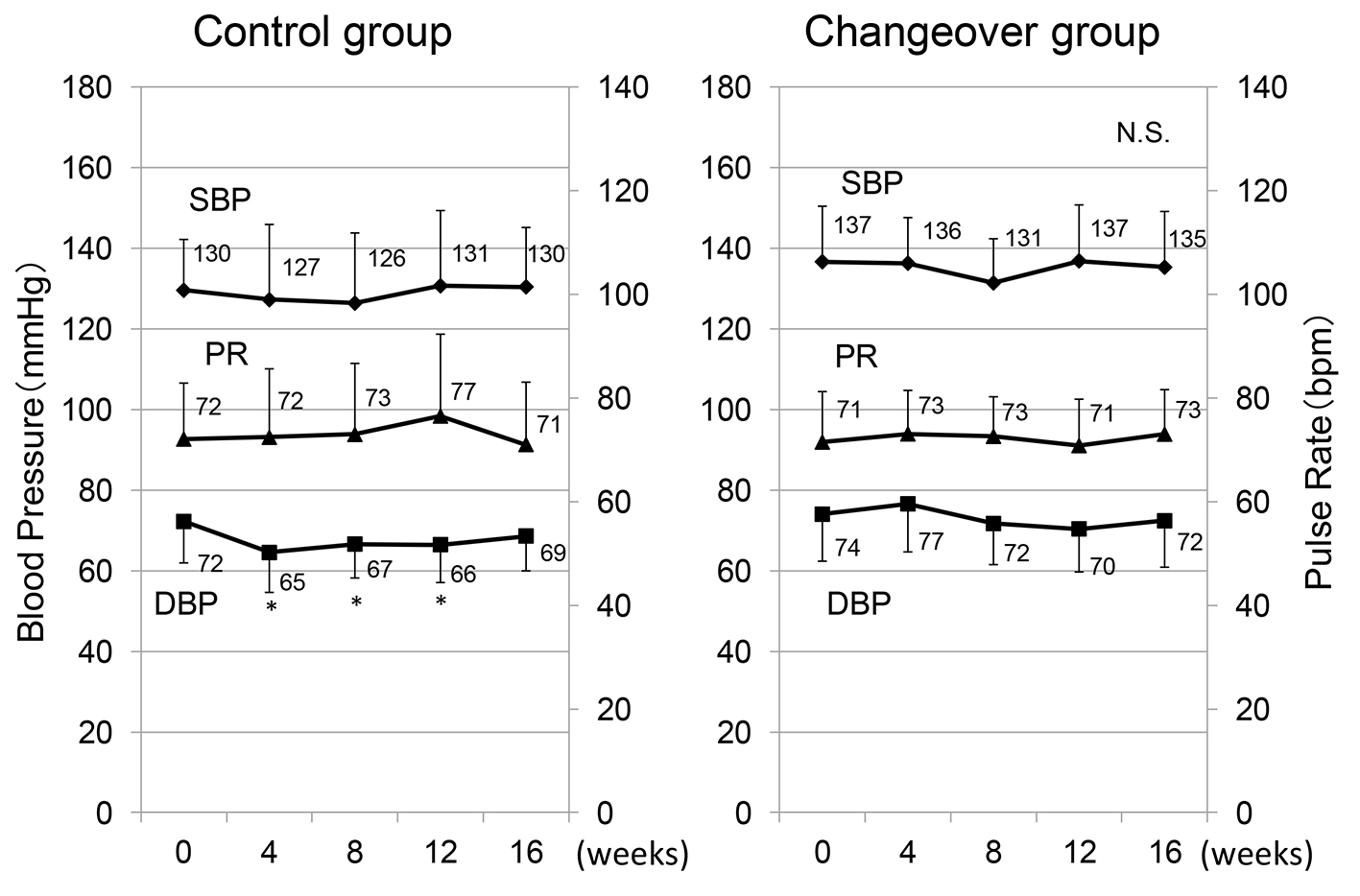 Click for large image | Figure 1. Time courses of systolic and diastolic blood pressure (SBP and DBP) and pulse rate (PR) in the control and changeover groups. *P < 0.05 vs. 0 weeks. |
Changes in biochemical parameters in the control and changeover groups
Biochemical parameters in blood at 0, 8 and 16 weeks in the control and changeover groups are shown in Table 2. There were no significant differences in the levels of biochemical parameters at 0 week between the groups, and there were no significant changes in biochemical parameters throughout the study period in both groups. Furthermore, no serious adverse effects were observed in any of the patients in this study.
 Click to view | Table 2. Change in Biochemical Parameters in the Control and Changeover Groups |
| Discussion | ▴Top |
In the present study, the depressor effect of a single-pill fixed-dose combination of azilsartan 20 mg/day and amlodipine 5 mg/day was comparable to that of combination of valsartan 80 mg/day or irbesartan 100 mg/day and amlodipine 5 mg/day. There were no significant changes in biochemical parameters throughout the study period.
We previously compared the efficacy and safety of valsartan and losartan [10, 11], irbesartan and olmesartan [12, 13] and azilsartan and olmesartan [14] in patients with HTN. Azilsartan medoxomil and azilsartan have been reported to have greater antihypertensive effects than other ARBs [5-8]. Thus, all ARBs may not have the same depressor effects, and azilsartan may have a stronger depressor effect than other ARBs. In this study, there were no significant differences in the time courses of BP throughout the study period in the control and changeover groups. In addition, although we used two combinations of ARBs and CCBs as prior single-pills, there were no significant differences in BP reduction with either combination of ARB and CCB (with a change from valsartan 80 mg/day + amlodipine 5 mg/day or irbesartan 100 mg/day + amlodipine 5 mg/day to azilsartan 20 mg/day + amlodipine 5 mg/day, BP changed from 135/73 to 135/73 mm Hg and from 140/76 to 135/71 mm Hg). ARBs did not appear to have differential depressor effects when they were combined with CCBs. There are several possible explanations for the lack of significant differences in BP reduction among the combination therapies with ARBs and CCBs. First, amlodipine has a relatively long elimination half-life of 35 - 45 h [15]. It has a relatively strong depressor effect because the depressor effect of nifedipine CR, which has the strongest depressor effect among CCBs, is comparable to that of amlodipine [16]. The differential depressor effect of ARBs may be masked by amlodipine, which has relatively strong and long-lasting depressor effects. Second, ARBs and CCBs are both effective antihypertensive agents with complementary mechanisms of action. Azilsartan did not appear to confer any benefit in ARB + CCB combination therapy, probably because combination therapy additively or synergistically induces an intensive BP-lowering effect.
This study has important limitations. First, the sample size was relatively small, which limits our ability to determine significance. Second, we applied a changeover design, where we switched from prior combinations of ARBs and CCBs to azilsartan and amlodipine. A crossover study would have been preferable. Third, DBPs in the control group were significantly decreased at 4, 8 and 12 weeks, although BP in the control group should not change because medications did not change through the study period. One possibility is due to seasonal variation of BP. However, the patients were randomly divided into two groups, and this may have minimized any difference in parameters.
In conclusion, the ability of a single-pill fixed-dose combination of azilsartan 20 mg/day and amlodipine 5 mg/day to reduce BP may be comparable to that of other combinations of ARBs and CCBs. No serious adverse effects were observed in any of the patients.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflicts of Interest
KS and SM have received grants and lecture honoraria from Daiichi-Sankyo Co. Ltd. KS is a Chief Director and SM is a Director of NPO Clinical and Applied Science, Fukuoka, Japan. KS has an Endowed “Department of Molecular Cardiovascular Therapeutics” supported by MSD, Co. Ltd. SM belongs to the Department of Molecular Cardiovascular Therapeutics, which is supported by MSD, Co. Ltd.
| References | ▴Top |
- Ogihara T, Kikuchi K, Matsuoka H, Fujita T, Higaki J, Horiuchi M, Imai Y, et al. The Japanese Society of Hypertension Guidelines for the Management of Hypertension (JSH 2009). Hypertens Res. 2009;32(1):3-107.
doi - Mancia G, De Backer G, Dominiczak A, Cifkova R, Fagard R, Germano G, Grassi G, et al. 2007 Guidelines for the Management of Arterial Hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens. 2007;25(6):1105-1187.
doi pubmed - Dahlof B, Sever PS, Poulter NR, Wedel H, Beevers DG, Caulfield M, Collins R, et al. Prevention of cardiovascular events with an antihypertensive regimen of amlodipine adding perindopril as required versus atenolol adding bendroflumethiazide as required, in the Anglo-Scandinavian Cardiac Outcomes Trial-Blood Pressure Lowering Arm (ASCOT-BPLA): a multicentre randomised controlled trial. Lancet. 2005;366(9489):895-906.
doi - Miura S, Saku K. Efficacy and safety of angiotensin II type 1 receptor blocker/calcium channel blocker combination therapy for hypertension: focus on a single-pill fixed-dose combination of valsartan and amlodipine. J Int Med Res. 2012;40(1):1-9.
doi pubmed - Rakugi H, Enya K, Sugiura K, Ikeda Y. Comparison of the efficacy and safety of azilsartan with that of candesartan cilexetil in Japanese patients with grade I-II essential hypertension: a randomized, double-blind clinical study. Hypertens Res. 2012;35(5):552-558.
doi pubmed - White WB, Weber MA, Sica D, Bakris GL, Perez A, Cao C, Kupfer S. Effects of the angiotensin receptor blocker azilsartan medoxomil versus olmesartan and valsartan on ambulatory and clinic blood pressure in patients with stages 1 and 2 hypertension. Hypertension. 2011;57(3):413-420.
doi pubmed - Bakris GL, Sica D, Weber M, White WB, Roberts A, Perez A, Cao C, et al. The comparative effects of azilsartan medoxomil and olmesartan on ambulatory and clinic blood pressure. J Clin Hypertens (Greenwich). 2011;13(2):81-88.
doi pubmed - Sica D, White WB, Weber MA, Bakris GL, Perez A, Cao C, Handley A, et al. Comparison of the novel angiotensin II receptor blocker azilsartan medoxomil vs valsartan by ambulatory blood pressure monitoring. J Clin Hypertens (Greenwich). 2011;13(7):467-472.
doi pubmed - Ojima M, Igata H, Tanaka M, Sakamoto H, Kuroita T, Kohara Y, Kubo K, et al. In vitro antagonistic properties of a new angiotensin type 1 receptor blocker, azilsartan, in receptor binding and function studies. J Pharmacol Exp Ther. 2011;336(3):801-808.
doi pubmed - Iwata A, Miura S, Imaizumi S, Kiya Y, Nishikawa H, Zhang B, Shimomura H, et al. Do valsartan and losartan have the same effects in the treatment of coronary artery disease? Circ J. 2007;71(1):32-38.
doi pubmed - Shiga Y, Miura S, Morii J, Kuwano T, Mitsutake R, Uehara Y, Inoue A, et al. Comparison of the efficacy and safety of single-pill fixed-dose combinations of losartan/hydrochlorothiazide and valsartan/hydrochlorothiazide in patients with hypertension (SALT-VAT study). Intern Med. 2011;50(21):2477-2483.
doi pubmed - Morii J, Miura S, Shiga Y, Sugihara M, Arimura T, Sako H, Zhang B, et al. Comparison of the efficacy and safety of irbesartan and olmesartan in patients with hypertension (EARTH study). Clin Exp Hypertens. 2012;34(5):342-349.
doi pubmed - Morii J, Miura S, Ike A, Shiga Y, Sugihara M, Iwata A, Kawamura A, et al. Comparison of the efficacies of irbesartan and olmesartan after successful coronary stent implantation. Intern Med. 2013;52(7):713-719.
doi pubmed - Adachi S, Miura S, Shiga Y, Arimura T, Kuwano T, Kitajima K, Ike A, et al. Depressor and Anti-Inflammatory Effects of Angiotensin II Receptor Blockers in Metabolic and/or Hypertensive Patients With Coronary Artery Disease: A Randomized, Prospective Study (DIAMOND Study). J Clin Med Res. 2016;8(10):743-748.
doi pubmed - Haria M, Wagstaff AJ. Amlodipine. A reappraisal of its pharmacological properties and therapeutic use in cardiovascular disease. Drugs. 1995;50(3):560-586.
doi pubmed - Tsutamoto T, Tsutsui T, Maeda K, Hayashi M, Wada A, Ohnishi M, Fujii M, et al. Effects of long-acting calcium channel antagonists on neurohumoral factors: comparison of nifedipine coat-core with amlodipine. J Cardiovasc Pharmacol. 2003;41(Suppl 1):S77-81.
pubmed
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Medicine Research is published by Elmer Press Inc.











