| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website http://www.jocmr.org |
Original Article
Volume 5, Number 1, February 2013, pages 49-56
An Aid to Decision-Making in Therapy of Retroperitoneal Fibrosis: Dynamic Enhancement Analysis of Gadolinium MRI
Alexander Sascha Brandta, c, Lars Kamperb, Sonja Kukukb, Werner Pirothb, Patrick Haageb, Stephan Rotha
aDepartments of Urology, Helios Klinikum Wuppertal, University of Witten/Herdecke, Wuppertal, Germany
bDiagnostic and Interventional Radiology, Helios Klinikum Wuppertal, University of Witten/Herdecke, Wuppertal, Germany
cCorresponding author: Alexander Sascha Brandt, Department of Urology, HELIOS Klinikum Wuppertal, University of Witten/Herdecke, Heusnerstr. 40, D-42285 Wuppertal, Germany
Manuscript accepted for publication December 10, 2012
Short title: Dynamic Enhancement Analysis of Gadolinium MRI
doi: https://doi.org/10.4021/jocmr1254e
| Abstract | ▴Top |
Background: Idiopathic retroperitoneal fibrosis (IRF) as an uncommon cause of obstructive uropathy is often primarily treated medically by the attending urologist. We evaluated dynamic enhancement analysis (DEA) as a possible predictor of response to medical treatment and for treatment monitoring.
Methods: From 2007, 24 patients with fibrosis were assessed by magnetic resonance imaging (MRI) with DEA. The dynamic enhancement quotient (DEQ) was measured before therapy with prednisone (n = 12) or tamoxifen (n = 12) and in follow-up investigations after 3 and 6 months. Response to medical treatment was recorded by changes in the retroperitoneal mass on MRI and possible relief of ureteral obstruction, which was monitored by intravenous pyelogram and/or MAG3 scan after removal of DJ stents.
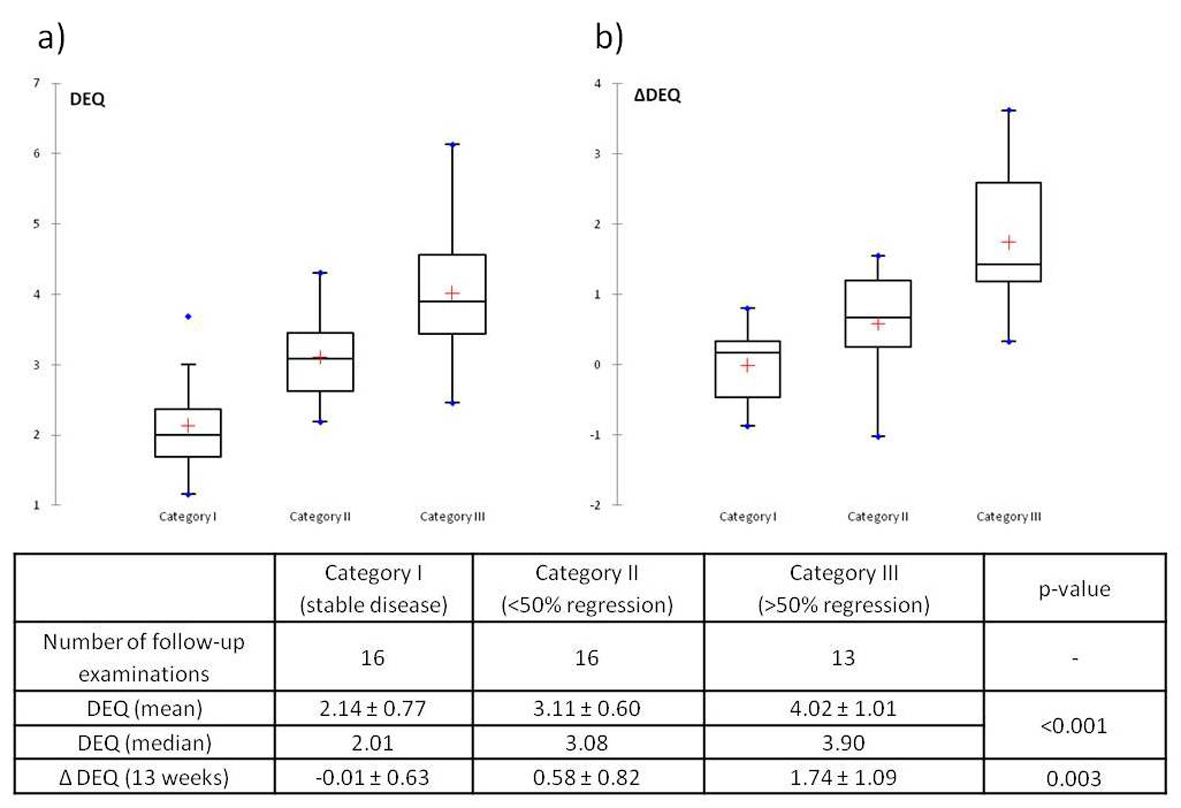
Results: Treatment groups did not differ significantly as to age, gender, or laboratory values, and response to medical treatment showed no significant difference between agents. Overall there were no cases of progression, 2 cases of stable disease, 11 cases of mild fibrotic regression, and 11 of significant or complete regression. DJ stents could successfully be removed in 21 of 35 renal units (60.0%). In a total of 61 DEAs the DEQ was significantly higher (P < 0.001) in patients with a good response (DEQ = 4.02) than in those with an average response (3.11) or none (2.14).
Conclusions: DEA was able to distinguish between patients with different response rates to medical treatment of IRF and may be useful to individualize therapeutic decision-making.
Keywords: Retroperitoneal fibrosis; Ormond’s disease
| Introduction | ▴Top |
Retroperitoneal fibrosis as an infrequent cause of obstructive uropathy was first described by Albarran in 1905 [1]. After the first report in English by Ormond in 1948, it became known as a self-contained disease, eponymously styled Ormond’s disease [2].
In more than two-thirds of patients, the cause remains unclear (idiopathic retroperitoneal fibrosis or IRF). In others, fibrosis occurs secondarily, for example, after medical or surgical treatment, infection, neoplasm, trauma, or radiotherapy [3, 4].
The diagnosis of IRF is often established primarily with computed tomography (CT) or magnetic resonance imaging (MRI); typically a retroperitoneal mass is evidenced surrounding the aorta from beneath the renal vessels to the aortic branch. With an atypical formation the diagnosis should be clarified histologically [3, 5].
Traditionally, the relief of urinary tract obstruction has been surgical, but at present the primary approach is often medical after initial relief with ureteral stents [6-8]. The goals of therapy are to remove the ureteral obstruction and to avert progression and recurrence of the fibrosis [3].
Attending physicians have to decide when to abandon medical treatment and perform surgical release of ureteral obstruction, but they can as yet rely only on parameters such as acute-phase reactants whose predictive value of a retroperitoneal mass decrease is uncertain [9].
Burn et al showed in 7 patients at a known disease stage that dynamic gadolinium enhancement in MRI was useful in differentiating newly diagnosed IRF from treated chronic disease [10]. We used this approach in follow-up investigations to study whether it could be used to differentiate between different response rates to medical treatment.
| Patients and Methods | ▴Top |
From April 2007 to March 2010, patients who were referred to our department with newly diagnosed IRF were examined with dynamic enhancement analysis (DEA). If need be, renal drainage was done by ureteral stenting; no patient had undergone medical therapy. Demographic, symptomatic, laboratory and radiographic data were recorded in the Else Kroner-Fresenius Registry of Retroperitoneal Fibrosis in Germany, a nationwide registry headquartered in our department [11].
Patients received initial MRI with dynamic gadolinium enhancement. After careful exclusion of malignant disease, medical therapy was begun with either prednisone or tamoxifen according to the contraindications for these agents and patient preference. Prednisone was given at a dose of 1 mg/kg body weight every second day for 10 weeks; thereafter 40 mg/day for 2 weeks, 20 mg/day for 2 weeks, 10 mg/day for 2 weeks, then 5 mg/day. Those receiving tamoxifen took 20 mg twice a day. Medical therapy was given for a total period of one year. All patients gave informed consent to treatment.
In follow-up examinations after 3, 6 and 12 months, response to treatment was evaluated by 3 independent observers (2 radiologists, 1 urologist) who assigned the change in retroperitoneal mass to one of four categories: (0) progression of disease, (I) stable disease, size reduction < 20%; (II) mild regression of fibrosis, reduction 20-50%; (III) significant or complete regression, reduction > 50% or no further delineable fibrosis. Dynamic gadolinium enhancement MRI was repeated in follow-up examinations after 3 and 6 months.
In cases of fibrosis regression and in accordance with the patient’s wish, DJ stents were removed and success was evaluated by intravenous pyelogram and/or MAG3 scan. After 6 and 12 months each case was reevaluated to decide whether to proceed with or change medical therapy or to perform surgery for ureteral obstruction. After successful medical or operative therapy patients were followed-up by MRI twice a year for the first and once a year afterwards.
Dynamic enhancement analysis in gadolinium-enhanced MRI
MRI was performed with a 1.5-Tesla scanner (Siemens MAGNETOM Avanto, Siemens Medical Systems, Erlangen, Germany) in combination with DEA to evaluate extent and activity of the IRF. Transverse and coronal standard T2-weighted images were acquired before injection of weight-adapted Gadoteridol (ProHance, Altana Pharma, Konstanz, Germany) and T1-weighted images before and after injection.
The T1-weighted DEA was performed in 13 repeated scans at the same table position in defined intervals: 7.5 sec between the first six and 17.5 sec between the last seven.
The dynamic enhancement was assessed in specific regions of interest within the IRF (ROI 1) and psoas muscle (ROI 2) with the “Mean-Curve” software package (Siemens Medical Systems, Erlangen, Germany), which generates curves of the dynamic intensities (Fig. 1a). The dynamic enhancement quotient (DEQ) was calculated after Burn and colleagues [10] by dividing the difference between the maximum enhancement and the pre-contrast intensity within the IRF and the psoas muscle (DEQ = Δ ROI 1 (IRF)/Δ ROI 2(psoas)).
 Click for large image | Figure 1. (a) After contrast injection, DEA recorded contrast enhancement of IRF (ROI 1) and psoas muscle (ROI 2), which generates typical curves of the dynamic intensities by the “Mean-Curve” software package. (b) DEA shows a high DEQ in a patient before the start of medical treatment with prednisone. (c) After 3 months the retroperitoneal mass is significantly reduced, as is the DEQ. (d) At 6 months the mass shows no further reduction and the DEQ is changed only slightly. |
In Category I (stable disease) there was almost no change in DEQ (ΔDEQ = -0.01 ± 0.63), whereas Categories II and III showed a regression of DEQ after treatment that was highest in Category III (Fig. 2b). The Kruskall-Wallis test again showed statistically significant differences between categories (P = 0.003), and the Wilcoxon rank-sum test showed statistical significance of ΔDEQ between categories I and III (P = 0.001) and II and III (P = 0.013), but not between categories I and II (P = 0.122).
| Discussion | ▴Top |
Idiopathic retroperitoneal fibrosis remains a disease rather seldom treated in urologic practice. Thus far the response to medical treatment has been assessable only by clinical tests such as regression of urinary obstruction or reduced size on imaging. Furthermore, there are no parameters able to predict whether medical therapy will be successful. Magrey et al showed that acute-phase reactants such as erythrocyte sedimentation rate and c-reactive protein at baseline are only poor predictors of a therapeutic response to glucocorticoid therapy [9]. For this reason the point at which to abandon medical treatment for surgical intervention is individual and nearly random.
It is reported that investigators typically use medical therapy as long as contrast uptake is evident in the retroperitoneal mass [12]. With DEA we were able to measure and objectify this uptake, whereas in a prior investigation we found that quotients of T1 and T2 signal intensities were not useful [13]. Therefore we think that DEA could be used to determine the right time for surgical intervention and could spare patients disappointing medical therapies with corresponding side effects.
In a series of 7 patients Burn et al showed for the first time that dynamic contrast-enhanced MRI can differentiate between newly diagnosed active fibrosis and treated chronic disease [10]. They based their findings on the different histopathologic appearance of IRF: in its early stage increased vascularity and vessel permeability associated with active inflammation [14] result in a higher local concentration of gadolinium; in later stages predominantly fibrous and collagenous tissue [14] allows a lower concentration.
In accordance with their approach, we used DEA in initial and follow-up examinations of patients receiving medical treatment for IRF. We observed different responses to medical treatment and found the DEQ to be a robust predictor: an above-average response to treatment appears to be associated with a higher DEQ than an average response or none, and differences were significant between groups. Additionally, a better response to medical treatment was associated with a greater decrease in contrast enhancement. In this way DEA, which has been used in other diseases, e.g. to differentiate between benign and malignant soft-tissue lesions [15] and to assess response in breast [16] and prostate cancer [17], could help individualize the medical treatment of IRF.
The advantage of MRI in combination with DEA, especially if multiple examinations are necessary, is that it is not associated with radiation as CT is. Additionally, MRI provides better contrast with surrounding retroperitoneal tissue and does not require iodinated contrast media [5]. A disadvantage is certainly that, because many patients with IRF suffer from renal insufficiency, the risk of gadolinium-associated nephrogenic systemic fibrosis must be carefully considered [18].
Several approaches to medical treatment have been described in the literature, but the lack of controlled trials has meant that treatment has not been standardized and is still largely empirical [7]. Because of the nonspecific inflammatory nature of IRF, corticosteroids are often used at onset [19]. Steroid therapy alone has shown sufficient regression of fibrosis, reported to be over 80% [20-24], but recurrence rates up to 25% have led several authors to propose steroid-sparing agents [25-28].
Tamoxifen has been described as a possible treatment in several anecdotal case reports since 1991 [29]. It seems to have anti-inflammatory or anti-fibroblastic activity in addition to its antiestrogenic effects [7]. Van Bommel et al in 2006 published the first extensive series with tamoxifen monotherapy in 19 patients and showed a slow but steady regression of the mass in 14 of 15 clinical responders with almost no side effects [29].
In our series we found regression of fibrosis in 22 of 24 patients (91.7%) after 6 months’ treatment, with no statistical significance between prednisone and tamoxifen (P = 0.761). Even though regression occurred in 91.7%, the ureteral stent could only be removed in 65.7% of renal units after 12 months. The other patients needed final surgical treatment.
Our series is limited by the small number of patients. Furthermore, patients were not randomized to therapy; their preference influenced the agent used. Therefore, further studies of treatment outcome with more patients and the presentation of long-term results are planned.
The evaluation with DEA is limited in patients with very small paravascular fibrotic plaques or with significant fibrotic regression because of restricted space for the manual positioning of ROIs. False results may occur if the ROI includes intravascular contrast enhancement owing to aortic pulsation, which led to exclusion of 9 patients in our series. Inhomogeneous contrast enhancement in different parts of the IRF may also limit the assessment of therapeutic response.
Upcoming studies must validate the method presented here in long-term follow-up to determine whether the DEQ can prevail as a safe and reliable predictor of therapeutic success.
Conclusion
Dynamic enhancement analysis was able to distinguish between patients with different response rates to medical treatment of retroperitoneal fibrosis. It appears that assessment of the dynamic enhancement quotient could be used to individualize medical treatment and to provide additional information for therapeutic decision-making: whether to continue medical therapy or to perform final surgical relief of ureteral obstruction. Additionally, therapy monitoring is possible for different therapeutic agents with DEA. Further investigations are mandatory to confirm that the DEQ can be a reliable predictor of response to medical treatment.
Acknowledgments
The storage of data of patients affected by retroperitoneal fibrosis in the Else Kroner-Fresenius Registry of Retroperitoneal Fibrosis is funded by the Else Kroner-Fresenius Stiftung (Foundation).
Conflict of Interest
The authors declare that they have no conflict of interest.
| References | ▴Top |
- Albarran J. Retention renale par peri-ureterite. Liberation externe de lutetere. Assoc Fr Urol. 1905;9:511.
- Ormond JK. Bilateral ureteral obstruction due to envelopment and compression by an inflammatory retroperitoneal process. J Urol. 1948;59(6):1072-1079.
pubmed - Vaglio A, Salvarani C, Buzio C. Retroperitoneal fibrosis. Lancet. 2006;367(9506):241-251.
pubmed - Fassina A, Boscolo Berto R, El Mazloum, Gottardo F, Artibani W. Retroperitoneal fibrosis after chemotherapy: part 2. Eur Urol. 2007;51(2):559-560.
pubmed - Arrive L, Hricak H, Tavares NJ, Miller TR. Malignant versus nonmalignant retroperitoneal fibrosis: differentiation with MR imaging. Radiology. 1989;172(1):139-143.
pubmed - Higgins PM, Bennett-Jones DN, Naish PF, Aber GM. Non-operative management of retroperitoneal fibrosis. Br J Surg. 1988;75(6):573-577.
pubmed - Swartz RD. Idiopathic retroperitoneal fibrosis: a review of the pathogenesis and approaches to treatment. Am J Kidney Dis. 2009;54(3):546-553.
pubmed - Wagenknecht LV, Hardy JC. Value of various treatments for retroperitoneal fibrosis. Eur Urol. 1981;7(4):193-200.
pubmed - Magrey MN, Husni ME, Kushner I, Calabrese LH. Do acute-phase reactants predict response to glucocorticoid therapy in retroperitoneal fibrosis? Arthritis Rheum. 2009;61(5):674-679.
pubmed - Burn PR, Singh S, Barbar S, Boustead G, King CM. Role of gadolinium-enhanced magnetic resonance imaging in retroperitoneal fibrosis. Can Assoc Radiol J. 2002;53(3):168-170.
pubmed - Brandt AS, Kamper L, Kukuk S, Haage P, Roth S. Associated findings and complications of retroperitoneal fibrosis in 204 patients: results of a urological registry. J Urol. 2011;185(2):526-531.
pubmed - Heidenreich A, Derakhshani P, Neubauer S, Krug B. [Treatment outcomes in primary and secondary retroperitoneal fibrosis]. Urologe A. 2000;39(2):141-148.
pubmed - Kamper L, Brandt AS, Scharwachter C, Kukuk S, Roth S, Haage P, Piroth W. MR evaluation of retroperitoneal fibrosis. Rofo. 2011;183(8):721-726.
pubmed - Mitchinson MJ. The pathology of idiopathic retroperitoneal fibrosis. J Clin Pathol. 1970;23(8):681-689.
pubmed - van Rijswijk CS, Geirnaerdt MJ, Hogendoorn PC, Taminiau AH, van Coevorden F, Zwinderman AH, Pope TL,
et al . Soft-tissue tumors: value of static and dynamic gadopentetate dimeglumine-enhanced MR imaging in prediction of malignancy. Radiology. 2004;233(2):493-502.
pubmed - Kuhl CK, Schild HH. Dynamic image interpretation of MRI of the breast. J Magn Reson Imaging. 2000;12(6):965-974.
pubmed - Alonzi R, Padhani AR, Allen C. Dynamic contrast enhanced MRI in prostate cancer. Eur J Radiol. 2007;63(3):335-350.
pubmed - Kribben A, Witzke O, Hillen U, Barkhausen J, Daul AE, Erbel R. Nephrogenic systemic fibrosis: pathogenesis, diagnosis, and therapy. J Am Coll Cardiol. 2009;53(18):1621-1628.
pubmed - Vaglio A, Palmisano A, Alberici F, Maggiore U, Ferretti S, Cobelli R, Ferrozzi F,
et al . Prednisone versus tamoxifen in patients with idiopathic retroperitoneal fibrosis: an open-label randomised controlled trial. Lancet. 2011;378(9788):338-346.
pubmed - Fry AC, Singh S, Gunda SS, Boustead GB, Hanbury DC, McNicholas TA, Farrington K. Successful use of steroids and ureteric stents in 24 patients with idiopathic retroperitoneal fibrosis: a retrospective study. Nephron Clin Pract. 2008;108(3):c213-220.
pubmed - Ilie CP, Pemberton RJ, Tolley DA. Idiopathic retroperitoneal fibrosis: the case for nonsurgical treatment. BJU Int. 2006;98(1):137-140.
pubmed - Kardar AH, Kattan S, Lindstedt E, Hanash K. Steroid therapy for idiopathic retroperitoneal fibrosis: dose and duration. J Urol. 2002;168(2):550-555.
pubmed - van Bommel EF, Siemes C, Hak LE, van der Veer SJ, Hendriksz TR. Long-term renal and patient outcome in idiopathic retroperitoneal fibrosis treated with prednisone. Am J Kidney Dis. 2007;49(5):615-625.
pubmed - van Bommel EF. Retroperitoneal fibrosis. Neth J Med. 2002;60(6):231-242.
pubmed - Marcolongo R, Tavolini IM, Laveder F, Busa M, Noventa F, Bassi P, Semenzato G. Immunosuppressive therapy for idiopathic retroperitoneal fibrosis: a retrospective analysis of 26 cases. Am J Med. 2004;116(3):194-197.
pubmed - Scheel PJ Jr., Piccini J, Rahman MH, Lawler L, Jarrett T. Combined prednisone and mycophenolate mofetil treatment for retroperitoneal fibrosis. J Urol. 2007;178(1):140-143, discussion 143-144.
pubmed - Swartz RD, Lake AM, Roberts WW, Faerber GJ, Wolf JS Jr. Idiopathic retroperitoneal fibrosis: a role for mycophenolate mofetil. Clin Nephrol. 2008;69(4):260-268.
pubmed - Warnatz K, Keskin AG, Uhl M, Scholz C, Katzenwadel A, Vaith P, Peter HH,
et al . Immunosuppressive treatment of chronic periaortitis: a retrospective study of 20 patients with chronic periaortitis and a review of the literature. Ann Rheum Dis. 2005;64(6):828-833.
pubmed - van Bommel EF, Hendriksz TR, Huiskes AW, Zeegers AG. Brief communication: tamoxifen therapy for nonmalignant retroperitoneal fibrosis. Ann Intern Med. 2006;144(2):101-106.
pubmed
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Medicine Research is published by Elmer Press Inc.











